Abstract
Advanced materials with various micro-/nanostructures have attracted plenty of attention for decades in energy storage devices such as rechargeable batteries (ion- or sulfur based batteries) and supercapacitors. To improve the electrochemical performance of batteries, it is uttermost important to develop advanced electrode materials. Moreover, the cathode material is also important that it restricts the efficiency and practical application of aluminum-ion batteries. Among the potential cathode materials, sulfur has become an important candidate material for aluminum-ion batteries cause of its considerable specific capacity. Two-dimensional materials are currently potential candidates as electrodes from lab-scale experiments to possible pragmatic theoretical studies. In this review, the fundamental principles, historical progress, latest developments, and major problems in Li–S and Al–S batteries are reviewed. Finally, future directions in terms of the experimental and theoretical applications have prospected.
Export citation and abstract BibTeX RIS
1. Introduction
Vast emission of CO2, which resulted in global climate change, has become one of the main problem of combustion reactions for past few centuries [1]. With this respect, clean and sustainable energy is a need for the development of future generations. As we face to history, the oldest lead-acid batteries were lithium-ion batteries (LIBs) which have been the focus of interest in applications of portable electronic devices such as laptops, tablets, cell phones, music players and wearable devices [2, 3]. Most of the LIBs are the state-of-the-art power sources for consumer electronics and they were operating mainly in the 4 V regime. Eglitis and Borstel were firstly found a new cathode material (Li2Co1Mn3O8) which can operate at around 5 V as predicted by first-principles methods [4, 5]. In order to enhance the efficiency of anode materials, alternative battery systems, such as lithium (Li) [6–12], sodium (Na) [13, 14], magnesium (Mg) [15, 16], and aluminum (Al) [17], have been attracting interest from the researchers [18–22]. Notably those materials are so rich in the Earth's crust that their usage is inexpensive.
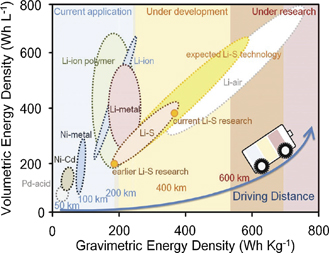
Recent studies revealed that sulfur-included lithium batteries (lithium–sulfur battery, Li–S) capable to provide an energy density of 500 Wh kg−1 which is much higher than that of Li-ion batteries (150–250 Wh kg−1) [23, 25, 26]. Moreover, these batteries can reduce the cost due to the use of sulfur atoms [27]. The other attractive choice for the sulfur-included battery is the aluminum sulfur (Al–S) battery, which is composed of an aluminum anode and sulfur cathode. Aluminum, the most abundant metallic element, can offer a high gravimetric capacity of 2980 mAh g−1 similar to lithium [28, 29]. In addition, a high gravimetric energy density of (almost 1340 Wh kg−1) can be achieved [30], which is over three-folds of a commercial LiCoO2-graphite cell [31]. So these sulfur-included batteries have recently gained increasing attention (figure 1) [31, 32].
Figure 1. Batteries for future market. [24] John Wiley & Sons. [Copyright © 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageMost of the reviews about sulfur-included batteries have focused on experimental studies [33–35]. Recently, theoretical studies on this subject have also become hotspot owing to high precision of simulations [36–38]. Therefore, our main interest is the recent theoretical and experimental studies on both Li–S and Al–S batteries concerning their storage capacities. In a nutshell, we would like to represent the results of experimental studies from existing literature to point out the efficiency of Li–S [39], and Al–S [40] batteries and mention about the advantages of these sulfur-based batteries. Researchers have been focused on developing electrode materials to construct advanced batteries, which in principle are needed to provide high capacity and high performance rate. Despite considerable progress have been achieved for those next-generation batteries, some existing problems or challenges are still in order.
The dimensional reduction has a significant effect on the demonstration of performbatteries. Materials of zero-dimension (0D) [41], one-dimension (1D) [42], two-dimensions (2D) [43] and three-dimensions (3D) materials [44, 45] have been widely considered for designing such batteries. Among these material groups, 0D and 1D structures provide low specific surface area, always a disadvantage for not only the electrochemical reaction of sulfur but also for the confinement of Li atoms on the surface of the material. In addition to this, 3D materials have been shown to possess high specific surface area, however, the modulation of the surface groups has difficulties arising from controlling the surface formation of the 3D structures. On the contrary, superior electron conductivity, high mechanical flexibility, large and suitable surface area for the chemical tunability of 2D materials have made them suitable materials for the research of electrochemical materials [46–48]. In addition, 2D materials are also potential candidates for the construction of functionalized flexible channels which are needed for the fast transfer of electrons. More importantly, 2D materials, exhibiting strong binding energy of Li and Al atoms, are suitable for capturing and catalyzing the redox reaction of Al- and Li–S batteries. Therefore, there is growing attention devoted to investigating the feasibility of 2D materials for Al- and Li–S battery applications.
We comprehensively discuss and highlight recent studies in detail. In conclusion, we would like to put forward predictions about practical applications that might pave the way to new technological developments. We hope that this review could be beneficial to both academic research and industrial commercialization of sulfur included Al and Li batteries. The latest improvements in the application of advanced batteries (Li–S and Al–S) were systematically analyzed in terms of their advantages and possible energy storage mechanisms and improvement strategies were discussed, in this review.
2. Experimental studies on metal–sulfur batteries
Recent experimental advances have demonstrated the systematic and precise synthesis of the building blocks of metal–sulfur batteries. While anode and cathode components, such as lithium and sulfide, have been synthesized using techniques such as sulfur reduction method, the analysis and the characterization of the synthesized materials have been analyzed using various techniques, namely XRD and Raman spectroscopy. For example, while XRD is very useful for characterizing of the crystal structure and chemical composition, Raman spectroscopy has often been used to identify the characteristic Raman peaks of polysulfides. In this section, experimental studies on metal–sulfur batteries are reviewed and discussed in detail.
2.1. Lithium sulfur batteries
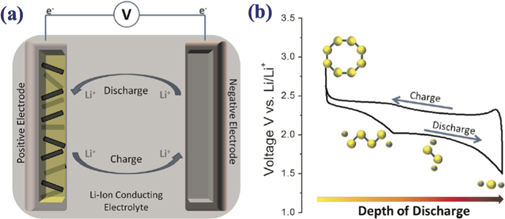
A Li–S battery consists of two main components, such as an anode (made of Li) and cathode (made of S) electrodes, which are separated by a separator and conductive electrolyte that can be either solid or liquid (for schematic illustration see figure 2(a)).
Figure 2. For a Li–S battery cell; (a) the working mechanism where the yellow part represents the sulfur cathode while black part stands for conductive (for example, carbon) part which is separated from the negative lithium electrode (gray) through an ion-conducting electrolyte. As the charging/discharging process goes on, lithium ions start to propagate through the electrolyte while electrons are accumulated through an external circuit. (b) Voltage-capacity graph corresponding to a Li–S cell where reduction of sulfur, S8 (yellow ring), by lithium (gray sphere), and its opening and shortening processes during discharge are shown. Note that this process continues until when the final discharge product of Li2S is created. Batteries for the future market. Reprinted with permission from [23]. Copyright (2013) American Chemical Society.
Download figure:
Standard image High-resolution imageSulfur is in the form of polyatomic molecules with various structures. The octasulfur with chemical formula S8 is an inorganic chemical and the most stable allotrope of sulfur at room temperature. During the charge–discharge process of the Li–S batteries, insoluble S8 undergoes a morphological and structural change and turns to insoluble lithium polysulfides such as Li2S2/Li2S and soluble Li2Sx (8 ⩽ x ⩽ 3) in a liquid electrolyte. However, these dissolved polysulfides make cycling travel between the anode and cathode while the charging process is in turn. The main problem of this shuttling is the polysulfides also involve the reoxidation reactions at the cathode and side reduction reactions with a lithium anode, which results in a poor cycle life that low battery efficiency has been observed. To deal with this problem using an ion-selective membrane can be a method to get rid of polysulfides shuttling for improving the electrochemical performance of the battery. Recently, Wang et al reported that using Li2S with a dual-phase electrolyte can hinder polysulfide migration and protect the lithium metal from direct contact with the electrolyte [49].
One of the main reasons of nonlinear charging/discharging of the sulfur-based batteries is the poor volumetric packing of sulfur, which gives rise to quick degradation of the battery arising from the induced mechanical stress. To solve this problem, researchers recommend to develop carbon based cathode composites and improving the conductivity. For this, increasing the sulfur loading of the cathode can be a solution. Manthiram et al, used carbon-cotton cathode in order to demonstrate stable Li–S batteries [50]. Carbon-cotton has many micro-/macroporous which allow to load high amount of active material due to having high surface area. Therefore, the carbon-cotton cathode has been demonstrated to exhibit enhanced cycle stability and improved cell-storage ability and even a high static capacity [50]. Recent studies showed that the effectiveness of the cathode can be related to the sulfur molecule size. For example, Xin et al has proved that using small sulfur allotropes such as S2, S3 and S4, avoided the polysulfide formation [51]. In addition, it was reported that LiS batteries can be important potential candidates with their high specific capacities, efficient cycling stabilities, and superior rate capabilities.
There are several studies for carbon based Li–S battery applications. Researchers aim to increase initial discharge capacity of the LiS batteries and also their cycling performance. Zhang et al, reported the synthesis of a new amorphous tantalum oxide, in which oxygen vacancies are embedded inside a microporous carbon matrix, as an electrocatalyst for the LiS batteries [52]. The oxygen vacancy in the tantalum oxide (Ta2O5−x ) changes the coordination number and electron band structure which increases the intrinsic conductivity and function as catalytic centers to accelerate LPS conversion. With this created oxygen vacancies and used microporous carbon matrix excellent Li–S performance such as long-term cyclability over 1000 cycles with an ultra-low capacity fading rate of 0.0295% per cycle has been obtained [52].
Zhan et al used carbon nanotube bucky paper and they deposited sulfur and Cox S electrocatalyst on it by a facile electrodeposition method to obtain a binder-free cathode material for LiS batteries [53]. Remarkably, this bucky paper gave high initial discharge capacity of 1650 mAh g−1 at 0.1 C. And for at current rate of 0.5 C, this synthesized cathode material has a capacity of 1420 mAh g−1 for the first cycle and reduces to 715 mAh g−1 after 500 cycles.
Gueon et al, showed the widely used sulfur/CS2 solution does not easily penetrate the hollow porous carbon sphere, due to the high interfacial energy of CS2. However, using a mixed solution of N-methyl-2-pyrrolidone (NMP) or isopropyl alcohol (IPA) and CS2 significantly improves the infiltration of the solution into the pores and resulting in complete sulfur encapsulation [54]. In addition, they found the control of sulfur loading such as the location of loading greatly affects the rate and reversibility of the sulfur transformation reaction and so the performance of the LiS battery.
Recently, Kim et al, prepared tungsten oxide/zirconia (WO3/ZrO2) and incorporated into a 2D compacted Li2S-graphene matrix through pelletization. By this way, direct conversion of Li2S to S8 observed due to high electrical conductivity of graphene and by the way tungsten oxide/zirconia particles behave as a polysulfide mediator to avoid the outflow of soluble polysulfide into the electrolyte solution [55].
Ultrathin polycrystalline bismuth nanosheets which have approximately of 4 nm thickness is developed as an electrocatalyst for polysulfide conversion by Xu et al [56]. This ultrathin bismuth LiS battery shows high reversible capacity of 408 mAh g−1 after 500 cycles at 10 C.
Successfully synthesized carbon fibers/Ti3C2Tx can be another potential candidate for commercial LiS battery devices. The interlayer of this material has three dimensional cross-linked conductive networks providing fast electron transfer channels and, thus, increase sulfur usage and save 'dead' sulfur. Jin et al, obtained high initial discharge capacity of 1380 mAh g−1 at a current density of 0.1 C and also carbon fibers/Ti3C2Tx has very low capacity loss of 0.044% per cycle within 1000 cycles at 1 C [57]. In addition, it presents a low self-discharge property which has proved by open circuit voltage (OCV) values. Jin et al presents that OCV retention is maintained as high as 97.8% after 200 h.
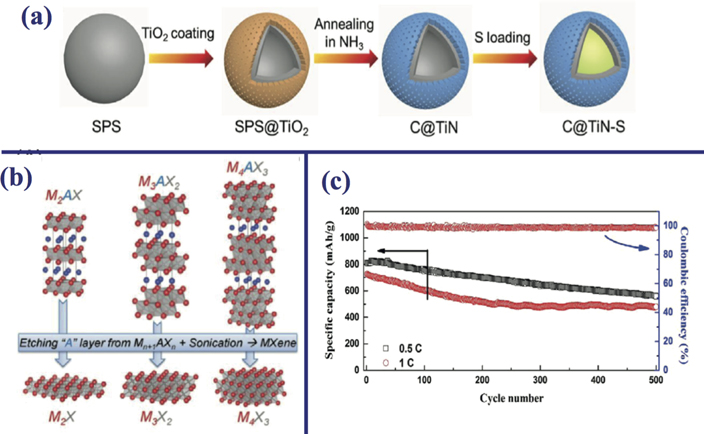
Wang et al demonstrated titanium included LiS battery using the following process [58]; as illustrated in figure 3(a), firstly the sulfonated hollow polystyrene spheres (SPS) were prepared and then by using sol–gel method SPS was covered with amorphous TiO2 layers. Following the annealing in a NH3 atmosphere, the formation of carbon mesoporous TiN dual-shell nanospheres (CTiN) were observed. Finally, sulfur melt diffusion method was used in order to obtain CTiN–S composites. It was mentioned in the report that CTiN dual-shell hollow nanospheres can improve the chemical adsorption, the physical confinement, and catalysis for sulfur species conversion. It was also reported that CTiN–S electrodes give rise to a high capacity of 820 mAh g−1 over 150 cycles at 0.2 C.
Figure 3. (a) Fabrication process of the C@TiN–S composites through different experimental stages. Reprinted from [58], Copyright (2019), with permission from Elsevier. (b) Crystal structures referring to layered MAX crystals with the ultra-thin forms of MXenes. Reproduced from [59]. CC BY 4.0. (c) For the rates of 0.5 and 1 C, the long-term cycling performance of Li–S batteries with the help of MPP separators. Reprinted with permission from [60]. Copyright (2016) American Chemical Society.
Download figure:
Standard image High-resolution imageTwo-dimensional MXenes materials (see figure 3(b)) have high conductivity and their layered structure reduces the surface volume ratio which is good for adsorption for adatom or molecule, and they have rich surface terminations. These properties make them ideal material for confining the polysulfide shuttling effects and allow one to use them for modifying separators and enhancing the electrochemical performance of LiS batteries. Song et al, improved the performance of LiS batteries by coating Ti3C2Tx MXene nanosheets (T is for O, OH, F atoms which are used for surface termination) on commercial 'Celgard' membrane. They reported that after coating the LiS battery with Ti3C2Tx MXene electrical conductivity is increased, so Li–S batteries including MXene-functionalized separators exhibit high discharge capacity of 1046.9 mAh g−1 at a low rate of 0.2 C. However, increasing of current density does not decrease the discharge capacity too much and exhibit discharge capacity of 743.7 mAh g−1 at a low rate of 1 C [60]. Other type of MXenes such as Mo2CTx or W2C are also used to improve LiS battery performance.
The electrode of Mo2CTx on carbon nanotube exhibits good electrochemical performances with regards to their high capacity (1314 mAh g−1), efficient rate capability and high initial reversible capacity for at 1.8 mg cm−2 sulfur loading and the capacity decreases to 959 mAh g−1 at 5.6 mg cm−2 sulfur loading [61]. Similarly, W2C clusters on the nitrogen–phosphorous co-doped carbon matrix (W2C/N/P-rGO) delivered high reversible capacities under different sulfur loadings. For example, discharge capacity of W2C/N/P-rGO at 3.1 mg cm−2 sulfur loading changes from ∼1200 mAh g−1 to ∼900 mAh g−1 with the increasing of cycling [62]. Detailed investigation and review about the two-dimensional MXenes for LiS batteries has been done by Zhang et al [59].
2.2. Aluminum sulfur batteries
The research on energy storage is mainly based on constructing abundant material-based batteries of low-cost exhibiting high energy density. Thus, recent research efforts have been focused to search for alternative materials such as Na, K, Ca, AL, and Mg for the construction of batteries. Among the abundant materials, aluminum is one of the most abundant element apart from oxygen and silicon and it has been used as an anode material in alkaline aqueous batteries for more than six decades [63]. Al metal has 3+ valence electrons that it can exchange three electrons during the electrochemical process in a battery system, which can result approximately in a higher volumetric capacity (8046 mAh cm−3 which is three times larger than that of lithium battery systems, 2062 mAh cm−3) [64]. Al is relatively stable in the atmospheric environment and easy to process, so it can improve the safety of chemical battery system and reduce the process cost.
To fulfill its potential, aluminum battery systems are creditable for accomplishing of either plating or stripping Al in ionic liquid electrolytes. Before the introduction of sulfur as a cathode in Al-based batteries, graphene, metal-oxides, and conductive polymers were used as cathode materials in rechargeable batteries. Besides a geographically well-distributed and streamlined production, sulfur with a high predicted specific capacity (1675 mAh g−1) [65], and volumetric capacity (3340 mAh cm−3) over a two-electron reduction process per atom and low cost has become an important research object of cathode materials for secondary batteries [29, 32, 39, 41]. The charge storage of S through a conversion reaction avoids the intercalation of bulk chloroaluminate ions, which are present in Al battery non-aqueous electrolytes. As a result, coupling of an Al anode with an S cathode in a battery provides a significant predicted energy density of 1371 Wh kg−1.
The schematic illustration of the aluminum–sulfur battery is shown in the left part of the figure 4(a), the mechanism of Al–S battery is based on conversion mechanism of S to Al2S3-like reduction process in series of polysulfide intermediate species. The reaction mechanism, during discharge/charge (see figure 4(b)), can be formulated using the equations on the anode (equation (1)), and the cathode (equation (2)) [66, 67].
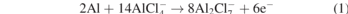
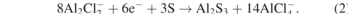
Apparently, the weak interaction of Al and S results in a high kinetic barrier and requires chloroaluminate ion bond breaking in order to let the aluminum ion to be free [68]. The right part of the figure 4(a) shows the charge–discharge curve of the aluminum–sulfur secondary battery. To prepare this battery, Wang et al [69] used nitrogen-doped three-dimensional hierarchical porous carbon/sulfur (N–C/S) composite as a positive electrode (cathode). According to their results, the performance of the aluminum sulfur battery which is based on N–C/S composite cathode material is very good. For instance, the first-lap discharge specific capacity is 1800 mAh g−1 and became almost half of this value after 10th cycles. However, this reducing becomes slow with the increasing of the cycles and as can be seen there is very small difference for the specific capacity values between the 15th and 20th cycles. At the same time, the specific capacity is still higher than 700 mAh g−1 after 20 cycles, which is high enough.
Figure 4. (a) Schematic diagram of Al–S battery (cathode and anode). Galvanostatic charge–discharge curves of the aluminum–sulfur secondary battery. [69] [2020], reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com). (b) Schematic illustration of the battery discharging process with the corresponding energy profiles standing for AcA coordinated with AlCl2 + through different sites (where Cl: green; O: red; Al: pink; C: gray; H: white; N: blue). Reprinted from [70], Copyright (2019), with permission from Elsevier. (c) Pouch cell-type of assembled ASB, including BN layer supported by S cathode; single layer BN represents a graphical illustration. Reproduced from [71]. CC BY 4.0. (d) The schematic diagram of energy profiles of the dissociation reactions of Al2Cl7- and Al2Cl6Br-anions. [68] John Wiley & Sons. [Copyright © 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageIn figure 4(c), illustration of the assembled pouch cell-type AlS batteries is shown where BN was used to support S cathode material [71]. Zhang et al, showed that two-dimensional materials such as BN, MoS2, and WS2 can fix S and sulfide compounds as the repeated charge/discharge processes proceeds. Note that, among the considered 2D materials, BN was demonstrated to display the highest capacity of 532 mAh g−1.
The first rechargeable Al–S battery that comprises EMIC-AlCl3 (1-ethyl-3-methylimidazolium chloride) ionic liquid electrolyte exhibited a high discharge capacity of 1500 mAh g−1 [30]. The demonstrated cell was shown to operate for only 20 cycles, therefore, its capacity was revealed to rapidly decay as result of S low electronic conductivity (5 × 10−28 Sm−1), which also results in low kinetics of the battery electrochemical reaction. Following, a rechargeable Al–S battery in an ionic-liquid electrolyte, in which an activated carbon cloth/sulfur was used as composite cathode, was successfully realized [67]. However, difficulties in the oxidation stage of AlSx
led to poor reversibility in Al/S chemistry. By confining the S species inside the microporous carbon pores of size less than 2 nm, it was shown to improve the electron access of S, prevent oxidation, increase the size of interfacial reaction area, and even reduce the diffusion length of Al3+ ions. Such process was reported to result in solid-state conversion reaction of S. By considering NBMPBr/AlCl3 (N-butyl-N-methylpiperidinium bromide) ionic liquid electrolyte, due to the lower dissociation of Al2Cl6Br− anions as compared to that of Al3
 , the weak kinetics of Al–S batteries can be improved [68]. However, even this approach could not get over the low cycling problem of the battery (see figure 4(d)). Very recently, composite structures of sulfur and single-walled carbon nanotubes having small diameter were constructed as a cathode building block for AlCl3: [EMIM]-based aluminum batteries in order to solve the poor stability and sluggish charge-storage problems [40]. Wu et al demonstrated rechargeable Al–selenium batteries (ASeBs) by using an eutectic solvent, thiourea-AlCl3, as an electrolyte. In their experiment, by a lowtemperature process, Se nanowires were grown directly on a flexible carbon cloth substrate (Se NWs@CC) and used as a cathode. The high electronic conductivity and low ionization potential of selenium (Se) was considered as its advantages for the improvement of the battery kinetics on electrochemical reaction and on the polarization reduction. In the final product of Se NWs@CC cathode, it was observed that the build material possesses a specific capacity of 260 mAh g−1 at 50 mAg−1, a sufficiently long cycling life (100 cycles) as well as a high Coulombic efficiency 93% at 100 mAg−1 [72].
, the weak kinetics of Al–S batteries can be improved [68]. However, even this approach could not get over the low cycling problem of the battery (see figure 4(d)). Very recently, composite structures of sulfur and single-walled carbon nanotubes having small diameter were constructed as a cathode building block for AlCl3: [EMIM]-based aluminum batteries in order to solve the poor stability and sluggish charge-storage problems [40]. Wu et al demonstrated rechargeable Al–selenium batteries (ASeBs) by using an eutectic solvent, thiourea-AlCl3, as an electrolyte. In their experiment, by a lowtemperature process, Se nanowires were grown directly on a flexible carbon cloth substrate (Se NWs@CC) and used as a cathode. The high electronic conductivity and low ionization potential of selenium (Se) was considered as its advantages for the improvement of the battery kinetics on electrochemical reaction and on the polarization reduction. In the final product of Se NWs@CC cathode, it was observed that the build material possesses a specific capacity of 260 mAh g−1 at 50 mAg−1, a sufficiently long cycling life (100 cycles) as well as a high Coulombic efficiency 93% at 100 mAg−1 [72].
3. Theoretical studies on metal–sulfur batteries
Theoretical and computational simulations, especially those performed on density functional formalism, have been shown to give almost reliable results that are compatible with experimental observations. While current experimental advances are devoted to demonstrating and developing of energy storage materials and battery systems, their working mechanisms should be clearly understood and investigated deeply for the industrial application step. Density functional theory (DFT) based simulations have been shown to be promising in energy storage, and significant contributions have been made to the understanding of mechanisms of electrochemical reactions. Moreover, DFT-based simulations can also offer excellent routes toward characterization techniques such as XRD and Raman measurements. In the present section, the contribution of DFT studies to the field of metal–sulfur batteries is presented.
3.1. Lithium sulfur batteries
Due to their low cost, predicted high capacity and energy density [73–75], Li–S batteries have gained great interest in various applications such as efficient energy storage technologies including portable electronic devices, hybrid/full electric vehicles, and large scale power grids [23, 76–78]. Several major technical obstacles, including dissolution of lithium polysulfides into liquid electrolyte, volume expansion of sulfur, and deposition of lithium sulfide, have been identified. For the past decade, investigation of an ideal cathode material for Li–S battery construction and enhancement of Li–S batteries for high-rate performance have become an important aim of the researchers. Accordingly, various nanostructured cathode materials have been developed including nanoporous carbon–sulfur composites, graphene–sulfur composites, one dimensional (1D) carbon–sulfur composites, conductive polymer–sulfur composites, porous oxide additives, and nanostructured Li2S cathodes [25]. These nanostructured sulfur cathodes have higher resistance against pulverization and exhibit quite fast reaction kinetics and a good trapping of soluble polysulfides. It has been reported that they have cycling capacity in the range of 568–1000 mAh g−1 for about 100–150 cycles of charge–discharge [79–84].
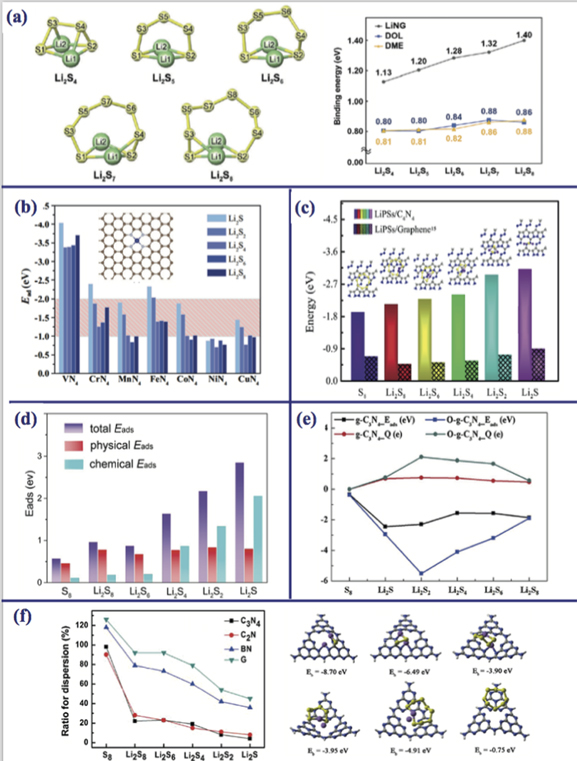
Li2S8, Li2S4, Li2S2, and Li2S that intrinsically have intermediate phase are significant products for the charge–discharge processes of Li–S batteries. Among these structures, Pan and Guan [85] investigated the four Li2S2 species with similar structural configurations (two different hexagonal structures with space groups P63/mmc and  , two different orthorhombic structures with space groups Cmca, and Immm). They have obtained the circuit voltages of orthorhombic (Immm), orthorhombic (Cmca), and hexagonal (P63/mmc) as 3.88, 3.95, and 3.91 respectively.
, two different orthorhombic structures with space groups Cmca, and Immm). They have obtained the circuit voltages of orthorhombic (Immm), orthorhombic (Cmca), and hexagonal (P63/mmc) as 3.88, 3.95, and 3.91 respectively.
Owing to their high surface to volume ratio and novel electronic properties, 2D materials present great promise as host materials to anchor lithium polysulfides (Li2Sx
). Zhao et al explore the adsorption and diffusion of various Li2Sx
(x = 1, 2, 3, 4, 6, 8) species on a phosphorene monolayer [86]. It was investigated that the diffusion mobility of Li2Sx
species having long chains (Li2S3, Li2S4, Li2S6, and Li2S8) is orientation-dependent and is much faster along the zigzag orientation as compared to armchair one. Among the Li2Sx
species, Li2S6 exhibit the smallest binding energy on phosphorene surface, a promising material for high-efficiency Li–S battery cathodes. In addition, Li et al [87] studied the effect of crystal structures of phosphorus polymorphs by considering different phosphorus allotropes (α-P, β-P, γ-P, δ-P,  -P, ζ-P, θ-P, and η-P). Their results showed that the crystal structures of phosphorus allotropes are very important for LiS batteries. Except for the
-P, ζ-P, θ-P, and η-P). Their results showed that the crystal structures of phosphorus allotropes are very important for LiS batteries. Except for the  -P, the remaining all other phosphorus allotropes can be suitable for the separations and modifications in Li–S batteries. In addition, α-P, β-P, γ-P, δ-P allotropes can be more convenient substrates for Li atom and Li–S diffusion in the sense that they result in lower barriers (please see figures 5(a)–(d)).
-P, the remaining all other phosphorus allotropes can be suitable for the separations and modifications in Li–S batteries. In addition, α-P, β-P, γ-P, δ-P allotropes can be more convenient substrates for Li atom and Li–S diffusion in the sense that they result in lower barriers (please see figures 5(a)–(d)).
Figure 5. (a) Structural configurations of adsorbed Li3Sx clusters on; α-P, η-P, ζ-P and θ-P. (b) Binding energies of Li2Sn species simulated on various phosphorene AMs (calculated by including vdW functional). Note that the Eb values of long-chain Li2Sn species and DOL/DME, which are the electrolyte solvent molecules, are plotted on the same graph for comparison. (c) vdW-free binding energies for Li2Sn species on various phosphorene AMs. (d) The vdW interaction ratio of different AMs. Adsorption configurations of Li2Sn clusters θ-P in the vdW-free assumption. Molecular structures of Li2Sn species corresponding to various lithiation amounts, ranging from unlithated S8 to Li2S (green and yellow balls represent lithium and sulfur atoms, respectively). Other colored balls all represent phosphorus to show the basic unit institutively. Reproduced from [87] with permission of The Royal Society of Chemistry. (e) Finally, the binding energies of lithium polysulfides for five different lithiation states (namely Li2S, Li2S2, Li2S4, Li2S6, Li2S8, S8) on the MX monolayers, ratio of van der Waals interactions for lithium polysulfides adsorption on MX monolayers and the most stable adsorption structures. [88] John Wiley & Sons. [Copyright © 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageIt has been reported in various studies that MXenes present a strong anchoring effect for soluble lithium polysulfide due to their smaller lattice constants and superior electrochemical performance. By combined experimental and theoretical studies, OH-functionalized MXene material and its defected species are explored as advantageous for entrapment of S in Li–S batteries [89]. Right after this study, Rao et al [90] obtained the interactions of lithium polysulfides (Li2Sx ) on Ti-based bare MXenes (Tin X(n−1); X = C, N; n = 2, 3, 4) and functionalized Ti2C with -F, -O, and -OH groups (Ti2C(OH)2, Ti2CO2, Ti2CF2). It has been reported that the strong bonding between Ti–S and also H–S dominate the interactions between MXenes and Li2Sx . It has also been investigated that the bare and OH-functionalized MXenes exhibits great electronic conductivity, which makes them feasible cathode materials to use in Li–S batteries with high performance. In addition to that, Lin et al [91] pointed out that O- and F-functionalized Ti2N present metallic character, which is advantageous for the redox reaction of Li2Sx species and the functionalized Ti2N possess good electrical conductivity that results in high rate performance of Li–S batteries. Afterwards, Li and et al [92] studied the binding energies of lithium (poly)sulfides on Ti2CO2, and M3C2O2 (M = Ti, V, Cr, Zr, Nb, Hf) MXenes, and also the behaviors of O-functionalized MXenes. Among these, Cr3C2O2 are found to exhibit the highest binding energy and the strongest anchoring effect. It was also reported that the lattice parameters of M3C2O2 MXenes and the binding energies are monotonically correlated. More explicitly, the binding energy value becomes higher as the lattice constant is decreased, and this results in an increase in anchoring effect towards lithium polysulfide species. Most recently, Wang et al [93] reported the adsorption energy of Li2Sn (n = 1–8) species on VS2 in the range of 1.13 eV–4.44 eV and the diffusion barrier energy of Li atom on VS2 as 0.25 eV, which implies the fast diffusion process.
Furthermore, black-phosphorene-like MX (M = Ge, Sn; X = S, Se) monolayers (group IV monochalcogenides) and graphene@MX heterostructures have been studied by Lv et al [88] as a functional host for Li–S batteries (please see figure 5(b)). They reported that MX-based heterostructures have enhanced electrical conductivity, great anchoring performance and fast charge/discharge rate for Li–S batteries. It has also been revealed that graphene@GeS heterostructure is a highly promising anchoring material for high-energy Li–S batteries. Chen et al systematically investigated the first-row transition metal sulfides (from Sc to Zn) as polar hosts for sulfur cathodes in LiS batteries to understand the general rationale for the host design of sulfur [95]. Their predictions revealed that diffusion barrier energy for lithium ion on vanadium sulfide (VS) is very low and VS has the strongest anchoring effects on Li2S immobilization among the other considered metal sulfides.
Yin et al [96] systematically explored the interaction mechanisms between N-doped graphene and lithium polysulfides by considering various doping configurations, including different types of N-dopants such as graphitic, pyridinic, pyrrolic and isolated/clustered amino. They unveiled that cluster of pyridinic N-dopants have stronger binding energies (1.23–3.57 eV) than electrolyte solvent molecules (DME and DOL) (0.87–0.98 eV) while isolated amino, graphitic, pyrrolic, or pyridinic N-dopant possess smaller or comparable binding energies (0.56–1.18 eV). Also, they reported that all the isolated N-dopants, except for graphitic N, show strong binding with Li polysulfides species comparing to piristine-G. An other nitrogen doped graphene is used by Yi et al [97], to investigate the adsorption energy of lithium polysulfides. They firstly adsorbed a Li atom on N-doped graphene and called this new structure as Li-trapped N-doped graphene and their results showed that the calculated binding energies of the lithium polysulfides on Li–N-graphene are in a range from 1.13 to 1.40 eV (please see figure 6(a)). The high binding energy which is more than 2.0 eV, is known to cause decomposition of the lithium polysulfides species. So, these obtained binding energies are lower than this critical point. Li2Sn (n = 1, 2, 4, 6, 8) lithium sulfides adsorbed on ideal graphene (IG) and N-doped graphenes which are N-substituted graphene (NG), pyridinic-like graphene (PIG), and pyrrolic-like graphene (PRG) by Rao et al [98]. Their results showed that the binding energies for Li2Sn changes from 0.94 eV to 3.17 eV and only IG + Li2Sn and NG + Li2Sn structures have lower binding energy values than 1.50 eV, all the other structures (PIG + Li2Sn and PRG + Li2Sn ) have higher than 2.50 eV binding energy. Single vacancy, divacancy or Stone–Wales (SW) type defected graphenes also investigated to understand can these structures be suitable for LiS battery applications [99, 100]. DFT calculations revealed that carbon vacancy site catches one S atom from the Li2Sn leading to the formation of a Li2Sn−1 molecule, which is weakly adsorbed on the created S-doped graphene. However, Li2Sn interaction with bare and SW defected graphene is almost similar [100].
Figure 6. (a) Optimized structures of the high-order lithium polysulfide (Li2Sx ) species. Green and yellow spheres represent the Li and S atoms, respectively. The right side of the panel shows the binding energies of the Li2Sx species with LiNG (Li-trapped N-doped graphene) are illustrated with gray circles, while blues squares depict for the Li2Sx species with DOL (1, 3-dioxolane). In addition, yellow triangles depict for the binding energies of the Li2Sx species with DME (1, 2-dimetylethane). Reproduced from [97] with permission of The Royal Society of Chemistry. (b) The adsorption energies (Ead) between Li2Sx (x = 1, 2, 4, 6, 8) and MN4@graphene (M = Cu, Ni, Co, Fe, Mn, Cr, V) surface with respect to the different lithiation stages. Reprinted from [101], Copyright (2018), with permission from Elsevier. (c) Eads for S8 cluster and Li2Sx on bare graphene (bars with patterns) and C4N4 monolayer (bars without patterns), respectively. The insets on the pillars show S8 or Li4Sn /C4N4 structures which are produced by the principle of minimum energy. Reproduced from [102] with permission of The Royal Society of Chemistry. (d) Physical and chemical contributions to the adsorption energy for the S8 cluster and Li4Sx (x = 1, 2, 4, 6, 8) species on monolayer BP. Reprinted from [94], Copyright (2019), with permission from Elsevier. (e) The adsorption energy (Eads) and depleted charge (Q) from S8 and Li2Sx (x = 1, 2, 4, 6, 8) to O-g-C3N4 and g-C3N4 for various lithiation stages, respectively. Reprinted from [103], Copyright (2018), with permission from Elsevier. (f) The right panel shows the most stable crystal structure of adsorbed S8 and LiPSs on C3N4. The left panel shows the ratio for dispersion interaction for G, C2N, C3N4 and BN at six different lithiation stages. Reprinted from [104], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution imageThe most favorable research strategy to find a suitable material for LiS battery is using graphene and improving its performance by external effects. Gong et al, found that heteroatom-modified graphene, h-G, (where h = B, N, O, P, or S) is effective on anchoring lithium polysulfides and on weakening the shuttle effect [105]. Wasalathilake et al, performed DFT calculations to investigate the interactions between the S8 molecule and long chain lithium polysulfides (Li2S4 and Li2S8) and graphene and graphene with decorated hydroxyl, epoxy and carboxyl groups [106]. Their calculations revealed that microporous graphene which is decorated with oxygen containing functional groups can successfully anchor S8, Li2S8 and Li2S4 moderate inter molecules through actions and this leads to the improvement of charge transfer and conductivity in the cathode side of Li–S batteries. Several studies showed that metal-N4 doped graphene sheets can be an efficient anchoring material for Li–S batteries. For this graphene sheet, the researchers firstly created porphyrin-like graphene by extracting one C–C bond and replacing the four C atoms facing to the divacancy site with four N atoms. At the end one metal atom is adding in the middle of N atoms. According to Zhang et al [101], DFT calculations for Li2Sn (n = 1, 2, 4, 6, 8) on the MN4@graphene (M = Cu, Ni, Co, Fe, Mn, Cr, V) showed that the binding energy values of Li2Sn on FeN4@graphene and CrN4@graphene is in an suitable interval (please see figure 6(b)) which indicates that these materials can be an anchor material. While the binding energies of Li2Sn for other co-doped systems such as MnN4@-graphene, CoN4@graphene, CuN4@graphene, and NiN4@graphene are less than 1 eV for the long chain lithium polysulfides which means these four MN4@graphene might not be an appropriate anchor material for the Li–S battery. Similar results (MN4@graphene + Li2Sn ) are obtained by other researchers [107, 108].
Theoretical studies were also focused on many carbon nitride or non-metallic monolayer materials to understand the interaction process of lithium polysulfides with these materials (please see figures 6(c)–(f)). Li et al studied the C4N4 monolayer and C4N4/graphyne heterostructure for LiS battery investigation. Theoretical calculations revealed that the adsorption energies of polysulfides on C4N4 in the range of 1.931 to 3.119 eV (please see figure 6(c)) and they showed that electrical conductivity of C4N4 can improve by creating C4N4/graphyne heterostructure [102]. He et al performed DFT calculations and obtained that oxygenated g-C3N4 give rise to a better electrical conductivity and adsorption energy of the Li2Sn species are higher than the pristine g-C3N4. Furthermore, they also confirmed these results experimentally and found that the O-g-C3N4 composite cathode possesses great electrochemical performance leading to a high reversible discharge capacity of 1030 mAh g−1 after 100 cycles at 0.2 C in Li–S batteries [103].
A comprehensive study is done by Zheng et al to understand the anchoring effect of non-metallic monolayers for lithium polysulfides in LiS batteries (please see figure 6(f)) [104]. They considered graphene, C2N, C3N4 and BN monolayers and found that C2N and C3N4 can provide suitable adsorption energies and enhance the redox kinetics. Other carbon included monolayers such as BC2N [109], C3B and C3N [110] are also investigated and found that except of C3N other two materials exhibit an excellent performance to anchor the lithium polysulfides cause of the strong chemical interaction (table 1).
Table 1. Energy barriers of Li atom, Li2Sn lithium polysulfides and S8 atom on 2D materials, the unit is in eV.
| Material | Li | Li2S | Li2S2 | Li2S4 | Li2S6 | Li2S8 | S8 | Reference |
|---|---|---|---|---|---|---|---|---|
| GeS (armchair) | 1.31 | 0.82 | 1.41 | 0.39 | 1.28 | 1.15 | [88] | |
| GeS (zigzag) | 0.50 | 0.83 | 0.89 | 0.90 | 0.90 | 0.88 | [88] | |
| GeSe (armchair) | 1.18 | 0.78 | 1.10 | 0.56 | 0.89 | 1.04 | [88] | |
| GeSe (zigzag) | 0.41 | 0.85 | 0.65 | 0.95 | 1.07 | 0.82 | [88] | |
| Phosphorene | 2.51 | 1.91 | -1.27 | 1.00 | 1.12 | [86] | ||
| SnS (armchair) | 0.97 | 0.40 | 0.47 | 0.49 | 0.52 | 0.69 | [88] | |
| SnS (zigzag) | 0.30 | 0.48 | 0.52 | 0.54 | 0.55 | 0.48 | [88] | |
| SnSe (armchair) | 1.07 | 0.69 | 0.52 | 0.62 | 0.75 | 0.98 | [88] | |
| SnSe (zigzag) | 0.23 | 0.61 | 0.65 | 0.68 | 0.78 | 0.80 | [88] | |
| Graphene@GeS (armchair) | 1.25 | 0.85 | 1.43 | 0.43 | 1.26 | 1.19 | [88] | |
| Graphene@GeS (zigzag) | 0.45 | 0.96 | 0.86 | 0.93 | 0.90 | 0.92 | [88] | |
| Ti2C | 5.82 | 8.87 | 15.41 | 22.71 | 29.73 | [90] | ||
| Ti2CF2 | 1.02 | 0.42 | 0.34 | [111] | ||||
| Ti2CO2 | 3.09 | 3.12 | 1.92 | 1.47 | 1.71 | 0.95 | [111] | |
| 2.02 | 1.80 | [90] | ||||||
| Ti2CO2 (bilayer) | 2.90 | 2.25 | 2.42 | 0.48 | [92] | |||
| Cr3C2O2 | 4.51 | 4.12 | 4.42 | 1.38 | [92] | |||
| V3C2O2 | 3.98 | 3.49 | 3.62 | 0.85 | [92] | |||
| Nb3C2O2 | 2.94 | 2.19 | 2.30 | 0.66 | [92] | |||
| Hf3C2O2 | 2.76 | 2.09 | 1.97 | 1.16 | [92] | |||
| Zr3C2O2 | 2.28 | 1.47 | 1.40 | 0.48 | [92] | |||
| Ti2COH2 | 3.43 | 4.90 | [90] | |||||
| Ti3C2 | 5.68 | 8.78 | 15.21 | 21.96 | 29.54 | [90] | ||
| Ti3C2F2 | 0.85 | 0.43 | 1.15 | [111] | ||||
| Ti3C2O2 | 3.21 | 2.61 | 2.92 | 0.51 | [92] | |||
| Ti4C3 | 5.78 | 8.94 | 15.04 | 23.26 | 30.70 | [90] | ||
| Ti2N | 6.09 | 9.16 | 15.85 | 22.47 | 29.18 | [90] | ||
| Ti2NF2 | 1.24 | 1.28 | 0.97 | 1.03 | 0.93 | 0.80 | [91] | |
| Ti2NO2 | 2.99 | 3.11 | 2.95 | 2.10 | 3.13 | 1.46 | [91] | |
| Ti3N2 | 6.13 | -9.21 | -15.59 | 22.62 | 29.33 | [90] | ||
| Ti4N3 | 5.97 | 9.10 | 15.59 | 22.82 | 29.50 | [90] | ||
| VS2 | 4.44 | 3.29 | 2.42 | 1.14 | 1.69 | 0.48 | [93] |
3.2. Aluminum sulfur batteries
Not only the limited number of experimental studies available, but also there were only a few reports describing theoretical studies on nonaqueous Al–S batteries [112]. The main problem could be the multivalence number of this metal ion. These types of multivalence metal ions such as Al, Ca, Mg are incompatible when used in LiS batteries with the typical organic liquid electrolytes. Therefore, the important thing for this kind of multivalence AlS batteries is finding proper electrolytes to effectively transport the aluminum ions and attain reversible plating/stripping of the anode metal species. Moreover, studies showed that the cathode volume expansion of AlS batteries after full discharging is too high (up to 272%) [34]. This exhibits that the designing of the cathode structure needs special attention. Here, theoretical studies will play a key role in order to understand complex electrochemical reactions through charge and discharge processes or finding suitable electrodes for AlS batteries. The characterization of surface and reduction mechanism can highlight by molecular dynamics (MD) simulation beyond DFT calculations in Al–S batteries. There are very few theoretical studies reported as of now. Very recently, Bhaurital et al observed a detailed charging and discharging processes in Al–S batteries by analysis of interfacial systems S8(001)/[EMIM]AlClS4 and Al2S3(001)/[EMIM]AlClS4-electrode, respectively [113] (please see figures 7(a)–(c)). The discharging process can occur through the continuous reduction of S to Al2S3-like products via a series of polysulfide intermedia species and involves the formation of various cationic and anionic intermediate species are observed. Hereby, they opened up a way to understand the complex electrochemical processes occurred in Al–S batteries. Chu et al showed that the energy barriers are key parameters for understanding the electrochemical reaction pathways on AlCl3/acetamide for reversible room-temperature Al–S battery which potentially presents a promising possibility for low-cost and high-performance energy storage system in future [70]. They reported that an initial capacity above 1500 mAh g−1 and good rate performance and [AlCl2(acetamide)2]+ ions led to an energetically favorable reaction pathway, which reveal the superior performance in comparison to the conventional AlCl4-based electrolyte.
Figure 7. (a)Structures used in the simulation of S8(001)/[EMIM]AlCl4-electrolyte and (b) Al2S3(001)/[EMIM]-AlCl4-electrolyte interfaces. (c) Discharging voltage versus the number of Al atoms which are added to the S8(001)/EMIM-AlCl4-electrolyte interface. (a)–(c) Reprinted with permission from [113]. Copyright (2020) American Chemical Society. (d) Radial distribution functions for S–S, Li–S and Al–S pairs as predicted from ab initio molecular dynamic simulations. (d) Reprinted from [114], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution imageAs mentioned in AlS experimental section, Yang et al used Al2Cl6Br− instead of Al2
 as the dissociation reaction reagent. Calculations based on DFT showed that the reaction rate of Al2Cl6Br− dissociation is 15 times faster than that of Al2
as the dissociation reaction reagent. Calculations based on DFT showed that the reaction rate of Al2Cl6Br− dissociation is 15 times faster than that of Al2
 , which is also verified by experimentally [68]. We believe that theoretical studies will be very important to improve the efficiency of Li/Al–S batteries.
, which is also verified by experimentally [68]. We believe that theoretical studies will be very important to improve the efficiency of Li/Al–S batteries.
Yu et al, performed ab initio molecular dynamics simulations based on DFT calculations for the amorphous Li3AlS3 structure [114]. The inset of figure 7(d) shows a snapshot of the amorphous Li3AlS3 structure obtained by the melt-quenching method while the plot for radial distribution functions between Al, Li and S atoms. As can be seen, the first peak position for Al–S pairs at 2.25 Å is close to the Al–S bond length (2.28) in crystalline Al2S3. The peak for Li–S pairs is located at 2.5, and it is very close to Li–S bond length in crystalline Li2S. However, the first peak for S–S pairs indicates that there are no polysulfides in the amorphous Li3AlS3 structure.
4. Conclusions
Energy storage has been one of the most important aspects of materials science and engineering over the past few decades. Almost over the past decade, there has been an increasing number of academic research on materials development, fundamental understanding and computational modeling, and application-based control algorithm developments using advanced materials having nanostructures. In addition to Li, the use of alternative structures, including Na, has been the hot spot due to limited lithium resources. It has been reviewed in the present article that sulfur-included Li-batteries provide higher energy density, almost three to five times, than that of ordered Li-ion batteries, which have limited applications. Using sulfur layer as the cathode material was shown to offer specific energy capacities on the order of 500 Wh kg−1, which was in the range of 150–200 Wh kg−1 for Li-ion batteries. In addition, aluminum, one of the most abundant metals, offers a high gravimetric capacity as that of Li. In addition to energy capabilities, the advantages of sulfur-based batteries are cheap and abundant. Even being non-toxic, they do not require top-up charging when being in the storage process.
In contrast to their advantages, the existing disadvantages, such as nonlinear charging and discharging, resulting in quick degradation of the battery due to mechanical stress, were demonstrated to be solved by developing carbon-based cathode composites and improving their conductivity. This was shown to result in the high-loading carbon cathode exhibiting enhanced cycle stability and improved cell-storage stability with a high static capacity. Moreover, in Al–S batteries, which were shown to operate for only 20 cycles and the capacity decays rapidly due to the low electronic conductivity of the sulfur, confinement of the sulfur species into microporous carbon was shown to improve the electron access of sulfur species, enlarge the interfacial reaction area resulting in the acceleration of battery reaction kinetics. In addition, among the materials used in sulfur-based batteries, two-dimensional materials have been widely demonstrated due to their high surface to volume ratio and intrinsic electronic properties. It was mentioned in our review that among different 2D materials, MXenes were shown to present a strong anchoring effect for soluble lithium polysulfide due to their smaller lattice constants and superior electrochemical performance. Furthermore, it was revealed in predictions that OH-functionalized MXenes exhibits excellent electronic conductivity, making them feasible for use as cathode materials, especially for Li–S batteries with high performance. Overall, the future applications of sulfur-based batteries can be visualized in three different areas, such as the need for more cycle life, the requirement for more power, and the need for both at the same time.
Briefly, this review article has shown that energy storage devices are the most important research area for researchers, and the amount of stored energy can increase with metal–sulfur batteries. The important question is which material is the best for the cathode material for these metal–sulfur batteries. Lithium–sulfur batteries can have a higher specific capacity than ordinary Li-ion batteries, and also aluminum–sulfur batteries can surpass this easily with their high capacities. Also, aluminum is cheaper and is more easily accessible than lithium. Consequently, to increase the capacities and the performance of these mentioned Al–S and Li–S batteries, increasing the number of studies is necessary. Especially, theoretical studies will play a critical role in this step because of their well matching results and predictions with the experimental results. So, there should be lots of theoretical studies before the experimental attempts. Many 2D materials such as group III–IV, group II–VI, TMDCs, TMTCs, GaSe, GaTe, etc are still waiting to be investigated to get an answer to the question 'are they suitable or not for the metal–sulfur batteries?'. In addition, how the possible defects or external effects will affect the batteries' performance and such questions should be answered by researchers. We hope that this review will be a good manual that summarizing this rapidly developing subject for the researchers who will make further fundamental contributions and seminal discoveries mediating battery applications.
Acknowledgments
BA acknowledges financial support from the Kırklareli University-BAP under Project No. 201. MY was supported by the Flemish Science Foundation (FWO-Vl) as a postdoctoral fellowship. FE is thankful to the scientific research projects of Aydın Adnan Menderes University, for financial support under the Project Number (FEF-20020).
Data availability statement
No new data were created or analysed in this study.