Abstract
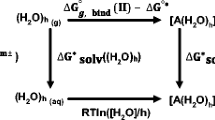
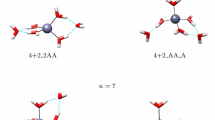
The reliability of ab initio methods to predict accurate thermodynamic properties and coordination geometries of mercury–thiolate complexes was examined with calculations at various levels of theory. The second-order Møller–Plesset perturbation theory (MP2) method in connection with the Stuttgart–Dresden–Bonn relativistic effective core potentials and the related correlation consistent valence basis set gives optimized Hg(RS) n model structures in good agreement with experimental data. Differences in thermodynamic stability among various models can be estimated with chemical precision using single-point energy calculation at the CCSD(T) level of theory performed on the MP2-optimized structures. This computational scheme was applied next to calculate the stability of aqueous linear (two coordinated), trigonal, and tetrahedral mercury–thiolate complexes. In alkaline solutions, the difference in complexation Gibbs free energy between the most stable (trigonal) and the less stable (tetrahedral) model complexes formed with free ligands is only −4.7 kcal mol−1. At neutral pH, the linear coordination is most stable. When the thiol ligands are structurally associated, as in biological systems, the trigonal coordination is most stable from pH 4.8 to 10.6. The relative stabilities of the three Hg–(RS) n bonding configurations reported herein can be further modified in biological environment by Hg-induced folding of proteins.



Similar content being viewed by others
References
Nomura A, Sugiura Y (2002) Contribution of individual zinc ligands to metal binding and peptide folding of zinc finger peptides. Inorg Chem 41:3693–3698
Lachenmann MJ, Ladbury JE, Dong J, Huang K, Carey P, Weiss MA (2004) Why zinc fingers prefer zinc: ligand-field symmetry and the hidden thermodynamics of metal ion selectivity. Biochemistry 43:13910–13925
Maret W, Valee BL (1998) Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci USA 95:3478–3482
Rosenzweig AC (2002) Metallochaperones: bind and deliver. Chem Biol 9:673–677
Robbins AH, McRee DE, Williamson M, Collett SA, Xuong NH, Furey WF, Wang BC, Stout CD (1991) Refined crystal structure of Cd, Zn metallothionein at 2.0 A resolution. J Mol Biol 221:1269–1293
Park JD, Liu Y, Klaassen CD (2001) Protective effect of metallothionein against the toxicity of cadmium and other metals(1). Toxicology 163:93–100
Fleischer H (2005) Structural chemistry of complexes of (n − 1)d10nsm metal ions with β-N-donor substituted thiolate ligands (m = 0, 2). Coord Chem Rev 249:799–827
Tang X-Y, Li H-X, Chen J-X, Ren Z-G, Lang J-P (2008) Synthetic and structural chemistry of groups 11 and 12 metal complexes of the zwitterionic ammonium thiolate ligands. Coord Chem Rev 252:2026–2049
Jalilehvand F, Leung B, Izadifard M, Damian E (2006) Mercury(II) cysteine complexes in alkaline aqueous solution. Inorg Chem 45:66–73
Mah V, Jalilehvand F (2008) Mercury(II) complex formation with glutathione in alkaline aqueous solution. J Biol Inorg Chem 13:541–553
Schicht O, Freisinger E (2009) Spectroscopic characterization of Cicer arietinum metallothionein 1. Inorg Chim Acta 362:714–724
Nagy KL, Manceau A, Gasper JD, Ryan JN, Aiken GR (2011) Metallothionein-like multinuclear clusters of mercury(II) and sulfur in peat. Environ Sci Technol 45:7298–7306
Cremer D, Kraka E, Filatov M (2008) Bonding in mercury molecules described by the normalized elimination of the small component and coupled cluster theory. ChemPhysChem 9:2510–2521
Filatov M, Cremer D (2004) Revision of the dissociation energies of mercury chalcogenides—unusual types of mercury bonding. ChemPhysChem 5:1547–1557
Asaduzzaman A, Khan M, Schreckenbach G, Wang F (2010) Computational studies of structural, electronic, spectroscopic and thermodynamic properties of methyl-mercury amino acid complexes and their Se analogues. Inorg Chem 49:870–878
Tossell JA (2001) Calculation of the structures, stabilities, and properties of mercury sulfide species in aqueous solution. J Phys Chem A 105:935–941
Watts J, Howell E, Merle JK (2013) Theoretical studies of complexes between Hg(II) ions and l-cysteinate amino acids. Int J Quantum Chem. doi:10.1002/qua.24565
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Woon DE, Dunning TH Jr (1993) Gaussian basis sets for use in correlated molecular calculations. III. The second row atoms, Al–Ar. J Chem Phys 98:1358–1371
Andrae D, Haeussermann U, Dolg M, Stoll H, Preuss H (1990) Energy-adjusted pseudopotentials for the second and third row transitions elements. Theor Chim Acta 77:123–141
Lim IS, Schwerdtfeger P, Metz B, Stoll H (2005) All-electron and relativistic pseudopotential studies for the group 1 element polarizabilities from K to element 119. J Chem Phys 122:104103
Martin JML, Sundermann A (2001) Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: the atoms Ga–Kr and In–Xe. J Chem Phys 114:3408–3420
Pantazis DA, Chen X-Y, Landis CR, Neese F (2008) All-electron scalar relativistic basis sets for third-row transition metal atoms. J Chem Theory Comput 4:908–919
Jansen G, Hess BA (1989) Revision of the Douglas–Kroll transformation. Phys Rev A 39:6016–6017
Douglas H, Kroll NM (1974) Quantum electrodynamical corrections to the fine structure of helium. Ann Phys 82:89–155
Hess BA (1986) Relativistic electronic-structure calculations employing a two-component no-pair formalism with external-field projection operators. Phys Rev A 33:3742–3748
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Cossi M, Scalmani G, Rega N, Barone V (2002) New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J Chem Phys 117:43–54
Raghavachari K, Pople JA (1978) Approximate fourth-order perturbation theory of the electron correlation energy. Int J Quantum Chem 14:91–100
Scuseria GE, Janssen CL, Schaefer HF III (1988) An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J Chem Phys 89:7382–7387
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:5968–5975
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem Phys Lett 157:479–483
Watts JD, Gauss J, Bartlett RJ (1993) Coupled-cluster methods with non-iterative triple excitations for restricted open-shell Hartree–Fock and other general single-determinant reference functions. Energies and analytical gradients. J Chem Phys 98:8718–8733
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford CT
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–557
Manceau A, Nagy KL (2008) Relationships between Hg(II)-S bond distance and Hg(II) coordination in thiolates. Dalton Trans 11:1421–1425
Kelly CP, Cramer CJ, Truhlar DG (2006) Aqueous solvation free energies of ions and ion-water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J Phys Chem B 110:16066–16081
Lennie A, Charnock J, Pattrick R (2003) Structure of mercury(II)-sulfur complexes by EXAFS spectroscopic measurements. Chem Geol 199:199–207
Mah V, Jalilehvand F (2010) Glutathione complex formation with mercury(II) in aqueous solution at physiological pH. Chem Res Toxicol 23:1815–1823
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5-Gibbs free energy of hydration at 298.15 K. J Chem Soc Faraday Trans 87:2995–2999
Svensson M, Humbel S, Morokuma K (1996) Energetics using the single point IMOMO (integrated molecular orbital molecular orbital) calculations: choices of computational levels and model system. J Chem Phys 105:3654–3661
Dapprich S, Komáromi I, Byun KS, Morokuma K, Frisch MJ (1999) A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J Mol Struct (Theochem) 462:1–21
Shoukry MM, Cheesman BV, Rabenstein DL (1988) Polarimetric and nuclear magnetic resonance studies of the complexation of mercury by thiols. Can J Chem 66:3184–3189
Ghosh D, Lee K-H, Demeler B, Pecoraro VL (2005) Linear free-energy analysis of mercury(II) and cadmium(II) binding to three-stranded coiled coils. Biochem 44:10732–10740
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648–5652
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Wernimont AK, Huffman DL, Lamb AL, O’Halloran TV, Rosenzweig AC (2000) Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol 7:766–771
Helmann JD, Ballard BT, Walsh CT (1990) The MerR metalloregulatory protein binds mercuric ion as a tricoordinate, metal-bridged dimer. Science 247:946–948
Watton SP, Wright JG, MacDonnell FM, Bryson JW, Sabat M, O’Halloran TV (1990) Trigonal mercuric complex of an aliphatic thiolate: a spectroscopic and structural model for the receptor site in the Hg(II) biosensor MerR. J Am Chem Soc 112:2824–2826
Hasnain SS (1988) Application of EXAFS to biochemical systems. Top Curr Chem 147:73–93
Jiang DT, Heald SM, Sham TK, Stillman MJ (1994) Structures of the cadmium, mercury, and zinc thiolate clusters in metallothionein: XAFS study of Zn7-MT, Cd7-MT, Hg7-MT, and Hg18-MT formed from rabbit liver metallothionein 2. J Am Chem Soc 116:11004–11013
Acknowledgments
The authors thank Isabelle Gautier-Luneau and Cyprien Lemouchi for fruitful discussions. This work was supported by the “Programme Blanc” of the French National Science Foundation (ANR) under Grant ANR-12-BS06-0008-01.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
214_2014_1457_MOESM1_ESM.doc
Online resources: ESM_1.doc contains the Gaussian 09 input files and the optimized structures of the mercury–thiolate and mercury–cysteine complexes. (DOC 61 kb)
Rights and permissions
About this article
Cite this article
Enescu, M., Manceau, A. High-level ab initio calculation of the stability of mercury–thiolate complexes. Theor Chem Acc 133, 1457 (2014). https://doi.org/10.1007/s00214-014-1457-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-014-1457-x