Abstract
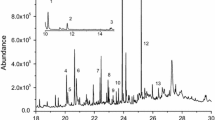
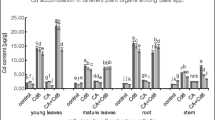
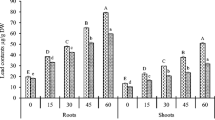
This work aims to investigate the nature and the specific mechanisms by which polycarboxylic compounds participate in the tolerance of Silene vulgaris to Cr with special attention given to the rhizosphere system. This knowledge is important to use this species in the implementation of phytoremediation technologies in Cr-polluted soils. According to the results, chromium is chelated and mobilized by the citric and malic acids in plant tissues, while oxalic acid might participate in the reduction and chelation of Cr in the rhizosphere. At the applied doses, the response of both exudation rate and root exudate composition (total polyphenols and quercitin) seems to involve a rearrangement in the lignification of the plant cell wall to immobilize Cr. Quercetin-3-dirhamnosyl-galactoside and apiin (apigenin-7-O-apiosyl-glucoside) have been identified as the major polyphenols in the root exudates of S. vulgaris. The increments found in the apiin concentration in root exudates seem to be related to the protection against Cr toxicity by chelation of Cr or by free radical scavenging. Though earlier response is detected in plant tissues, results from this work together with previous studies in S. vulgaris indicate that exudation might be a regulated mechanism of protection under Cr exposition in S. vulgaris that may involve mainly Cr reduction and chelation.


Similar content being viewed by others
References
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76. doi:10.1016/j.plantsci.2012.07.014
Arnetoli M, Vooijs R, ten Bookum W, Galardi F, Gonnelli C, Gabbrielli R, Schat H, Verkleij JAC (2008) Arsenate tolerance in Silene paradoxa does not rely on phytochelatin-dependent sequestration. Environ Pollut 152:585–591. doi:10.1021/jf072203d
Bartlett RJ (1991) Chromium cycling in soils and water-links, gaps, and methods. Environ Health Perspect 92:17–24. doi:10.2307/3431133
Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329:1–25. doi:10.1007/s11104-009-0266-9
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L, Renella G, Landi L, Nannipieri P (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol. Fertility. Soils 48:123–149. doi:10.1007/s00374-011-0653-2
Chan ZL, Qin GZ, XB X, Li BQ, Tian SP (2007) Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J Proteome 6:1677–1688. doi:10.1021/pr060483r
Choppala G, Bolan N, Park JE (2013) Chromium contamination and its risk management in complex environmental settings. Adv Agron 120:129–172. doi:10.1016/B978-0-12-407686-0.00002-6
Dhal B, Thatoi HN, Das NN, Pandey BD (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 250-251:272–291. doi:10.1016/j.jhazmat.2013.01.048
Díaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161(1):179–188. doi:10.1016/S0168-9452(01)00410-1
Drzewiecka K, Mleczek M, Gasecka M, Magdziak Z, Golinski P (2012) Changes in Salix viminalis L. cv. ‘Cannabina’ morphology and physiology in response tonickel ions—hydroponic investigations. J Hazard Mater 217:429–438. doi:10.1016/j.jhazmat.2012.03.056
Garcia-Gonzalo P, Pradas del Real AE, Lobo MC, Perez-Sanz A (2016) Different genotypes of Silene vulgaris (Moench) Garcke grown on chromium-contaminated soils influence root organic acid composition and rhizosphere bacterial communities. Environ Sci Pollut Res 1–12. doi:10.1007/s11356-016-6667-4
Gatto MG, Ippolito A, Linsalata V, Cascarano NA, Nigro F, Vanadia S, Di Venerea D (2011) Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol Tec 61:72–82. doi:10.1016/j.postharvbio.2011.02.005
James BR, Bartlett RJ (1983) Behavior of chromium in soils: V. Fate of organically complexed Cr (III) added to soil. J Environ Qual 12: 169-172. doi:10.2134/jeq1983.00472425001200020003
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jung C, Maeder V, Funk F, Frey B, Sticher H, Frossard E (2003) Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 252:301–312. doi:10.1023/a:1024775803759
Kasthuri J, Rajendiran N (2009) Functionalization of silver and gold nanoparticles using amino acid conjugated bile salts with tunable longitudinal plasmon resonance. Colloids and Surfaces B-Biointerfaces 73:387–393. doi:10.1016/j.colsurfb.2009.06.012
Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352. doi:10.1093/jexbot/52.359.1339
Kidd P, Barcelo J, Pilar Bernal M, Navari-Izzo F, Poschenrieder C, Shilev S, Clernente R, Monterroso C (2009) Trace element behaviour at the root-soil interface: implications in phytoremediation. Environ Exp Bot 67:243–259. doi:10.1016/j.envexpbot.2009.06.013
Kupper H, Mijovilovich A, Meyer-Klaucke W, Kroneck P (2004) Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol 134:748–757. doi:10.1104/pp.103.032953
Kutrowska A, Szelag M (2014) Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol Plant 36:1957–1968. doi:10.1007/s11738-014-1576-y
Laguerre M, Lecomte J, Villeneuve P (2007) Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. Prog Lipid Res 46:244–282. doi:10.1016/j.plipres.2007.05.002
Loponen J, Lempa K, Ossipov V, Kozlov MV, Girs A, Hangasmaa K, Haukioja E, Pihlaja K (2001) Patterns in content of phenolic compounds in leaves of mountain birches along a strong pollution gradient. Chemosphere 45(3):291–301. doi:10.1016/S0045-6535(00)00545-2
Luo Z, Wadhawan A, Bouwer EJ (2010) Sorption behaviour of nine chromium(III) organic complexes in soil. Int J Environ Sci Tec 7:1–10. doi:10.1007/BF03326111
Magdziak Z, Kozlowska M, Kaczmarek Z, Mleczek M, Chadzinikolau T, Drzewiecka K, Golinski P (2011) Influence of Ca/Mg ratio on phytoextraction properties of Salix viminalis. II. Secretion of low molecular weight organic acids to the rhizosphere. Ecotoxicol Environ Saf 74:33–40. doi:10.1016/j.ecoenv.2010.09.003
Makoi JHJR, Ndakidemi PA (2007) Biological, ecological and agronomic significance of plantphenolic compounds in rhizosphere of the symbiotic legumes. Afr Biotechnol 6:1358–1368
Marschner P, Neumann G, Kania A, Weiskopf L, Lieberei R (2002) Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin. Plant Soil 24:6167–6174. doi:10.1023/A:1020663909890
Meier S, Alvear M, Borie F, Aguilera P, Ginocchio R, Cornejo P (2012) Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol Environ Saf 75:8–15. doi:10.1016/j.ecoenv.2011.08.029
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Mikhaeil BR, Badria F, Maatooq G, Amer M (2004) Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch C 59:468–476. doi:10.1515/znc-2004-7-803
Olko A, Abratowska A, Zylkowska J, Wierzbicka M, Tukiendorf A (2008) Armeria maritima from a calamine heap—initial studies on physiologic-metabolic adaptations to metal-enriched soil. Ecotoxicol Environ Saf 69:209–218. doi:10.1016/j.ecoenv.2007.01.010
Omololu PA, Rocha JBT, Kade IJ (2011) Attachment of rhamnosyl glucoside on quercetin confers potent iron-chelating ability on its antioxidant properties. Exp Toxicol Pathol 63:249–255. doi:10.1016/j.etp.2010.01.002
Papp JF (2015) Chromium. U.S. Geological Survey, Mineral Commodity Summaries, January 2015. Available from: http://minerals.usgs.gov/minerals/pubs/mcs/2015/mcs2015.pdf. Accessed 17.4.15
Pradas del Real AE, Garcia-Gonzalo P, Alarcon R, Gonzalez-Rodriguez A, Lobo MC, Perez-Sanz A (2013a) Effect of genotype, Cr(III) and Cr(VI) on plant growth and micronutrient status in Silene vulgaris (Moench). Span J Agric Res 11:685–694. doi:10.5424/sjar/2013113-3536
Pradas del Real AE, Lobo MC, Gil-Díaz M, Pérez-Sanz A (2013b) Study of Cr + 6 behavior in three agricultural soils, E3S Web of Conferences. EDP Sciences, p 08008. doi:10.1051/e3conf/20130108008
Pradas del Real AE, Lobo MC, Perez-Sanz A, McNear DH (2014a) The chromium detoxification pathway in the multimetal accumulator Silene vulgaris. Environ Sci Technol 48(19):11479–11486. doi:10.1021/es502099g
Pradas del Real AE, Garcia-Gonzalo P, Lobo MC, Perez-Sanz A (2014b) Chromium speciation modifies root exudation in two genotypes of Silene vulgaris. Environ Exp Bot 104:1–6. doi:10.1016/j.envexpbot.2014.05.002
Pradas del Real AE, Garcia-Gonzalo P, Gil-diaz M, Gonzalez-Rodriguez A, Lobo MC, Perez-Sanz A (2016) Eco-physiological response of S. vulgaris to Cr(VI): influence of concentration and genotype. Int J Phytoremediat 18(6):567–574
Puzon G, Roberts A, Kramer D, Xun L (2005) Formation of soluble organo-chromium(III) complexes after chromate reduction in the presence of cellular organics. Environ Sci Technol 39:2811–2817. doi:10.1021/es048967g
Rao AS (1990) Root flavonoids. Bot Rev 56:1–84. doi:10.1007/BF02858531
Rengel Z (2002) Genetic control of root exudation. Plant Soil 245:59–70. doi:10.1023/a:1020646011229
Sarret G, Willems G, Isaure M, Marcus MA, Fakra SC, Frerot H, Pairis S, Geoffroy N, Manceau A, Saumitou-Laprade P (2009) Zinc distribution and speciation in Arabidopsis halleri x Arabidopsis lyrata progenies presenting various zinc accumulation capacities RID A-1549-2010 RID A-2499-2009. New Phytol 184:581–595. doi:10.1111/j.1469-8137
Schmidt BM, Erdman JW, Lila MA (2005) Effects of food processing on blueberry antiproliferation and antioxidant activity. J Food Sci 70:389–394. doi:10.1111/j.1365-2621
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254. doi:10.1007/s10311-013-0407-5
Singh A, Jain A, Sarma BK, Upadhyay RS, Singh HB (2014) Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol Res 169:353–360. doi:10.1016/j.micres.2013.09.014
Sobrino-Plata J, Herrero J, Carrasco-Gil S, Pérez-Sanz A, Lobo C, Escobar C, Millán R, Hernández LE (2013) Specific stress responses to cadmium, arsenic and mercury appear in the metallophyte Silene vulgaris when grown hydroponically. RSC Adv 3:4736–4744. doi:10.1039/C3RA40357B
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999. doi:10.1007/s11738-012-1169-6
USEPA (1992) Method 7196a. Chromium Hexavalent (Colorimetric), Available from: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/7196a.pdf. Accessed 15.10.14
USEPA (1998) Toxicological review of hexavalent chromium. U.S. Goverment Printing Office, Washington
USEPA (U.S. Environmental Protection Agency) (2013) National Priorities List (NPL) at http://www.epa.gov/superfund/sites/query/queryhtm/nplfin.htm
Wenzel WW (2009) Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 321:385–408. doi:10.1007/s11104-008-9686-1
Weston LA, Mathesius U (2013) Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol 39:283–297. doi:10.1007/s10886-013-0248-5
Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, Liu X (2012) Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol 196:125–138. doi:10.1111/j.1469-8137.2012.04236.x
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant soil 249:139–156.
Zhao F, McGrath S, Crosland A (1994) Comparison of 3 wet digestion methods for the determination of plant sulfur by inductively-coupled plasma-atomic emission-spectroscopy (ICP AES). Commun Soil Sci Plant Anal 25:407–418. doi:10.1080/00103629409369047
Acknowledgements
We are grateful to the Analysis Service Unit facilities of ICTAN for the analysis of chromatography and mass spectrometry. The authors thank the financial supports provided by EIADES (S2009/AMB-1478, Comunidad de Madrid), RTA-000150-00-00-INIA and “Contratación de doctores 2007 INIA-CCAA” and MINECO AGL2012-30803. We thank “Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario” for the fellowship support of Ana E. Pradas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yi-ping Chen
Electronic supplementary material
ESM 1
(DOCX 93 kb)
Rights and permissions
About this article
Cite this article
Pradas del Real, A.E., Silvan, J.M., de Pascual-Teresa, S. et al. Role of the polycarboxylic compounds in the response of Silene vulgaris to chromium. Environ Sci Pollut Res 24, 5746–5756 (2017). https://doi.org/10.1007/s11356-016-8218-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8218-4