Abstract
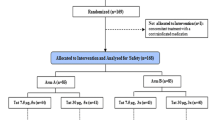
In this multicenter, randomized, double-blind study the activity of polyI:polyC12U administered with zidovudine was evaluated in the treatment of HIV infection. Thirty-six HIV-positive, pre-AIDS individuals (100–500 CD4+ cells/mm3) who had had at least six months of zidovudine therapy received polyI:polyC12U (400 or 700 mg) or placebo twice weekly with zidovudine. PolyI:polyC12U subjects with baseline CD4+ counts≥300/mm3 showed a trend towards reduced CD4+ loss versus placebo recipients. PolyI:polyC12U subjects were more likely to exhibit positive delayed-type hypersensitivity responses than placebo recipients. Placebo subjects crossing over to polyI:polyC12U therapy demonstrated improved CD4+ and delayed-type hypersensitivity responses. PolyI: polyC12U subjects with baseline CD4+ counts≥300/mm3 were less likely to develop AIDS than similar placebo subjects. PolyI:polyC12U therapy of HIV-positive subjects restored or stabilized immune function as indexed by delayed-type hypersensitivity reactivity and, in individuals with CD4+ counts>300/mm3, abrogated CD4+ loss and reduced disease progression. PolyI:polyC12U was generally well-tolerated in this zidovudine-treated population. No subject discontinued therapy due to an adverse reaction or aberrant laboratory parameter.
Similar content being viewed by others
References
Carter WA, Pitha PM, Marshall LW, Taxawa I, Ts'o POP: Structural requirements of the rln-rCn complex for induction of human Interferon. Journal of Molecular Biology 1972, 70: 567–587.
Ts'o POP, Alderfer JL, Levy J, Marshall LW, O'Malley J, Horoszewicz JS, Carter WA: An integrated and comparative study of the antiviral effects and other biological properties of the polyinosinic acid-polycytidylic acid and its mismatched analogues. Molecular Pharmacology 1976, 12: 299–312.
Milhaud PG, Machy P, Colote S, Lebleu B, Leserman L: Free and liposome-encapsulated double-stranded RNAs as inducers of interferon, interleukin-6 and cellular toxicity. Journal of Interferon Research 1991, 11: 261–265.
Ushijima H, Rytik PG, Schäcke H, Scheffer U, Müller WEG, Schröder HC: Mode of action of the anti-AIDS compound poly(I):poly(C12U) (Ampligen): Activator of 2′,5′-oligoadenylate synthetase and double-stranded RNA-dependent kinase. Journal of Interferon Research 1993, 13: 161–171.
Montefiori DC, Mitchell WM: Antiviral activity of mismatched double-stranded RNA against human immunodeficiency virus in vitro. Proceedings of the National Academy of Sciences of the United States of America 1987, 84: 2985–2989.
Montefiori DC, Robinson WE, Mitchell WM: In vitro evaluation of mismatched double-stranded RNA (Ampligen) for combination therapy in the treatment of acquired immunodeficiency syndrome. AIDS Research and Human Retroviruses 1989, 5: 193–203.
O'Marro SD, Armstrong JA, Asuncion C, Gueverra L, Ho M: The effect of combinations of Ampligen and zidovudine or dideoxyinosine against human immunodeficiency viruses in vitro. Antiviral Research 1992, 17: 169–177.
Laurent-Crawford AG, Krust B, Deschamps de Paibuste E, Montagnier L. Hovanessian AG: Antiviral action of polyadenylic-polyuridylic acid against HIV in cell cultures. AIDS Research and Human Retroviruses 1992, 8: 285–290.
Carter WA, Ventura D, Shapiro DE, Strayer DR, Gillespie DH, Hubbell HR: Mismatched double-stranded RNA, ampligen (polyl: polyC12U) demonstrates antiviral and immunostimulatory activities in HIV disease. International Journal of Immunopharmacology 1991, 13, Supplement 1: 69–76.
Armstrong JA, McMahon D, Huang XL, Pazin GJ, Gupta P, Rinaldo CR, Schoenfeld DA, Gaccione P, Tripoli CA, Bensasi S, Ho M: A Phase I study of ampligen in human immunodeficiency virus-infected subjects. Journal of Infectious Diseases 1992, 166: 717–722.
Carter WA, Strayer DR, Brodsky I, Lewin M, Pellegrino MG, Einck L, Henriques HF, Simon GL, Parenti DM, Scheib RG, Schulof RS, Montefiori DC, Robinson WE, Mitchell WM, Volsky DJ, Paul D, Paxton H, Meyer WA, Kariko K, Reichenbach N, Suhadolnik RJ, Gillespie DH: Clinical, immunological and virological effects of Ampligen, a mismatched dsRNA, in patients with AIDS or AIDS-related complex. Lancet 1987, i: 1286–1292.
Strayer DR, Carter WA, Pequignot E, Topolsky D, Brodsky I, Suhadolnik RJ, Reichenbach N, Paul D, Einck L, Hubbell HR, Pinto A, Strauss K, Gillespie D: Activity of a synthetic dsRNA — Ampligen — in HIV disease. Clinical Biotechnology 1991, 3: 169–175.
Kornbluth RS, McCutchan JA: Skin test responses as predictors of tuberculosis infection and of progression in HIV-infected persons. Annals of Internal Medicine 1993, 119: 241–243.
Centers for Disease Control and Prevention: Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality Weekly Review 1993, 41, No. RR17: 1–19.
Runyon RP: Fundamentals of statistics in the biological, medical, and health sciences, Duxbury Press, Boston, 1985.
Birx DL, Brundage J, Larson K, Engler R, Smith L, Squire E, Carpenter G, Sullivan M, Rhoads J, Oster C, James W, Lupton G, Wierzba T, Burke D, Redfield R, the Military Medical Consortium for Applied Retroviral Research: The prognostic utility of delayed-type hypersensitivity skin testing in the evaluation of HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes 1993, 6: 1248–1257.
Blatt SP, Hendrix CW, Butzin CA, Freeman TM, Ward WW, Hensley RE, Melcher GP, Donovan DJ, Boswell RN: Delayed-type hypersensitivity skin testing predicts progression to AIDS in HIV-infected patients. Annals of Internal Medicine 1993, 119: 177–184.
Choi S, Lagakos SW, Schooley TR, Volberding PA: CD4+ lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Annals of Internal Medicine 1993, 118: 674–680.
Concorde Coordinating Committee: Concorde: MRC/-ANRS randomized double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Lancet 1994, 343: 871–881.
Gordin FM, Hartigan PM, Klimas NG, Zolla-Pazner SB, Simberkoff MS, Hamilton JD, for the Department of Veterans Affairs Cooperative Study Group: Delayed-type hypersensitivity skin tests are an independent predictor of human immunodeficiency virus disease progression. Journal of Infectious Diseases 1994, 169: 893–894.
Clerici M, Shearer GM: A TH1 → TH2 switch is a critical step in the etiology of HIV infection. Immunology Today 1993, 14: 107–111.
Manetti R, Annunziato F, Tomasevic L, Giannó V, Parronchi P, Romagnani S, Maggi E: Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-α and interleukin-12. European Journal of Immunology 1995, 25: 2656–2660.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thompson, K.A., Strayer, D.R., Salvato, P.D. et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:PolyC12U in the treatment of HIV infection. Eur. J. Clin. Microbiol. Infect. Dis. 15, 580–587 (1996). https://doi.org/10.1007/BF01709367
Issue Date:
DOI: https://doi.org/10.1007/BF01709367