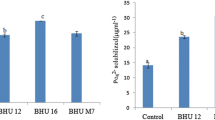
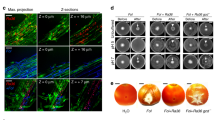
Abstract
Plant rhizosphere and internal tissues may constitute a relevant habitat for soil bacteria displaying high catabolic versatility towards xenobiotic aromatic compounds. Root exudates contain various molecules that are structurally related to aromatic xenobiotics and have been shown to stimulate bacterial degradation of aromatic pollutants in the rhizosphere. The ability to degrade specific aromatic components of root exudates could thus provide versatile catabolic bacteria with an advantage for rhizosphere colonization and growth. In this work, Cupriavidus pinatubonensis JMP134, a well-known aromatic compound degrader (including the herbicide 2,4-dichlorophenoxyacetate, 2,4-D), was shown to stably colonize Arabidopsis thaliana and Acacia caven plants both at the rhizoplane and endorhizosphere levels and to use root exudates as a sole carbon and energy source. No deleterious effects were detected on these colonized plants. When a toxic concentration of 2,4-D was applied to colonized A. caven, a marked resistance was induced in the plant, showing that strain JMP134 was both metabolically active and potentially beneficial to its host. The role for the β-ketoadipate aromatic degradation pathway during plant root colonization by C. pinatubonensis JMP134 was investigated by gene inactivation. A C. pinatubonensis mutant derivative strain displayed a reduced ability to catabolise root exudates isolated from either plant host. In this mutant strain, a lower competence in the rhizosphere of A. caven was also shown, both in gnotobiotic in vitro cultures and in plant/soil microcosms.




Similar content being viewed by others
References
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Barret CF, Parker MA (2006) Coexistence of Burkholderia, Cupriavidus, and Rhizobium sp. nodule bacteria on two Mimosa spp. in Costa Rica. Appl Environ Microbiol 72:1198–1206
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Chao CY, Yin MC (2009) Antibacterial effects of Roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog Dis 6:201–206
Chen WM, de Faria SM, Straliotto R, Pitard RM, Simoes-Araujo JL, Chou J-H, Chou Y-J, Barrios E, Prescott AR, Elliott GN, Sprent JI, Young JPW, James EK (2005) Proof that Burkholderia strains form effective symbioses with legumes: a study of novel mimosa-nodulating strains from South America. Appl Environ Microbiol 71:7461–7471
Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Ait Barka E (2005) Endophytic colonization of Vitis vinifera L. by a plant growth-promoting bacterium, Burkholderia sp. strain PsJN. Appl Environ Microbiol 71:1685–1693
Costa R, Salles JF, Berg G, Smalla K (2006) Cultivation-independent analysis of Pseudomonas species in soil and in the rhizosphere of field-grown Verticillium dahliae host plants. Environ Microbiol 8:2136–2149
Diouf D, Samba-Mbaye R, Lesueur D, Ba AT, Dreyfus B, de Lajudie P, Neyra M (2007) Genetic diversity of Acacia seyal Del. rhizobial populations indigenous to Senegalese soils in relation to salinity and pH of the sampling sites. Microb Ecol 54:553–566
Dorn E, Hellwig M, Reineke W, Knackmuss HJ (1974) Isolation and characterization of a 3-chlorobenzoate-degrading Pseudomonad. Arch Microbiol 99:61–70
Elliott GN, Chou JH, Chen WM, Bloemberg GV, Bontemps C, Martínez-Romero E, Velázquez E, Young JP, Sprent JI, James EK (2009) Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ Microbiol 11:762–778
Espinosa-Urgel M, Salido A, Ramos JL (2000) Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J Bacteriol 182:2363–2369
Gough C, Galera C, Vasse J, Webster G, Cocking EC, Dénarié J (1997) Specific flavonoids promote intercellular root colonization of Arabidopsis thaliana by Azorhizobium caulinodans ORS571. Mol Plant Microbe Interact 10:560–570
Graham TL (1998) Flavonoid and flavonol glycoside metabolism in Arabidopsis. Plant Physiol Biochem 36:134–144
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Harwood CS, Parales RE (1996) The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 50:553–590
Hoque MS, Broadhurst LM, Thrall PH (2011) Genetic characterization of root-nodule bacteria associated with Acacia salicina and A. stenophylla (Mimosaceae) across south-eastern Australia. Int J Syst Evol Microbiol 61:299–309
Jain V, Gupta K (2003) The flavonoid naringenin enhances intercellular colonization of rice roots by Azorhizobium caulinodans. Biol Fertil Soil 38:119–123
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg BJ (2006) Organic acids, sugars, and l-tryptophan in exudates of vegetables growing on stone wool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19:250–256
Kiely P, Haynes J, Higgins C, Franks A, Mark G, Morrissey J, O’Gara F (2006) Exploiting new systems-based strategies to elucidate plant-bacterial interactions in the rhizosphere. Microb Ecol 51:257–266
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Kuiper I, Bloemberg GV, Lugtenberg BJ (2001) Selection of a plant-bacterium pair as a novel tool for rhizostimulation of polycyclic aromatic hydrocarbon-degrading bacteria. Mol Plant Microbe Interact 10:1197–1205
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Lugtenberg BJ, Kravchenko LV, Simons M (1999) Tomato seed and root exudates sugars: composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ Microbiol 1:429–436
Lugtenberg BJ, Chin-A-Woeng TF, Bloemberg GV (2002) Microbe–plant interactions: principles and mechanisms. Antonie van Leeuwenhoek 81:373–383
Lykidis A, Pérez-Pantoja D, Ledger T, Mavromatis K, Anderson IJ, Ivanova NN, Hooper SD, Lapidus A, Lucas S, González B, Kyrpides NC (2010) The complete multipartite genome sequence of Cupriavidus necator JMP134, a versatile pollutant degrader. PLoS One 5(3):e9729
Marx JM, Lidstrom ME (2002) Broad-host-range cre-lox system for antibiotic marker recycling in Gram-negative bacteria. Biotechniques 33:1–6
Mathysse AG, Stretton S, Dandie C, McClure NC, Goodman AE (1996) Construction of GFP vectors for use in gram-negative bacteria other than Escherichia coli. FEMS Microbiol Lett 145:87–94
Matilla MA, Espinosa-Urgel M, Rodríguez-Herva JJ, Ramos JL, Ramos-González MI (2007) Genomic analysis reveals the major driving forces of bacterial life in the rhizosphere. Genome Biol 8:R179
Mendes R, Pizzirani-Kleiner AA, Araujo WL, Raaijmakers JM (2007) Diversity of cultivated endophytic bacteria from sugarcane: genetic and biochemical characterization of Burkholderia cepacia complex isolates. Appl Environ Microbiol 73:7259–7267
Mercado-Blanco J, Bakker PA (2007) Interactions between plants and beneficial Pseudomonas spp.: exploiting bacterial traits for crop protection. Antonie Van Leeuwenhoek 92:367–389
Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant–microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol 132:146–153
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomoie 23:375–396
O’Callaghan K, Stone P, Hu X, Griffiths D, Davey M, Cocking E (2000) Effects of glucosinolates and flavonoids in colonization of the roots of Brassica napus by Azorhizobium caulinodans ORS571. Appl Environ Microbiol 66:2185–2191
Pérez-Pantoja D, De la Iglesia R, Pieper DH, González B (2008) Metabolic reconstruction of aromatic compounds degradation from the genome of the amazing pollutant degrading bacterium Cupriavidus necator JMP134. FEMS Microbiol Rev 32:736–794
Pérez-Pantoja D, Donoso R, Junca H, González B, Pieper DH (2010a) Phylogenomics of aerobic bacterial degradation of aromatics, Chap. 39. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology, vol 2. Springer, Berlin, pp 1356–1397
Pérez-Pantoja D, González B, Pieper DH (2010b) Aerobic degradation of aromatic hydrocarbons, Chap. 4. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology, vol 2. Springer, Berlin, pp 800–837
Pérez-Pantoja D, Donoso RA, Agulló L, Córdova M, Seeger M, Pieper DH, González B (2011) Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales. Environ Microbiol. doi:10.1111/j.1462-2920.2011.02613.x
Porra R, Thompson W, Kriedmann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta 975:384–394
Ramos-González MI, Campos MJ, Ramos JL (2005) Analysis of Pseudomonas putida KT2440 gene expression in the maize rhizosphere: in vitro expression technology capture and identification of root-activated promoters. J Bacteriol 187:4033–4041
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278:1–9
Ryu C-M, Hu C-H, Locy RD, Kloepper J (2005) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 268:285–292
Schnitzler JP, Madlung J, Rose A, Seitz HU (1992) Biosynthesis of p-hydroxybenzoic acid in elicitor-treated carrot cell cultures. Planta 188:594–600
Shaw LJ, Burns RG (2003) Biodegradation of organic pollutants in the rhizosphere. Adv Appl Microbiol 53:1–60
Shaw LJ, Burns RG (2004) Enhanced mineralization of [U-14C]2, 4-dichlorophenoxyacetic acid in soil from the rhizosphere of Trifolium pratense. Appl Environ Microbiol 70:4766–4774
Shaw LJ, Burns RG (2005) Rhizodeposition and the enhanced mineralization of 2, 4-dichlorophenoxyacetic acid in soil from the Trifolium pratense rhizosphere. Environ Microbiol 7:191–202
Shaw LJ, Morris P, Hooker JE (2006) Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ Microbiol 8:1867–1880
Simons M, van der Biji AJ, Brand I, de Wager LE, Wijffelman CA, Lugtenberg BJ (1996) Gnotobiotic system for studying rhizosphere colonization by plant growth promoting Pseudomonas bacteria. Mol Plant Microbe Interact 9:600–607
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Tan J, Bednarek P, Liu J, Schneider B, Svatos A, Hahlbrock K (2004) Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry 65:691–699
Uren NC (2000) Types, amounts and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere, biochemistry and organic substances at the soil–plant interface. Marcel Dekker, New York, pp 19–40
Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM (2003) Metabolic profiling of root exudates of Arabidopsis thaliana. J Agric Food Chem 51:2548–2554
Webster G, Jain V, Davey M, Gough C, Vasse J, Denarie J, Cocking E (1998) The flavonoid naringenin stimulates the intercellular colonization of wheat roots by Azorhizobium caulinodans. Plant Cell Environ 21:373–383
Acknowledgments
This work was funded by the FONDECYT grants 3090051, 1070343, and 1110850, and the Millennium Nucleus on “Plant Functional Genomics” grant P/06-009-F. This study is also part of the research program FONDAP 1501-0001 funded by CONICYT to the Center for Advanced Studies in Ecology and Biodiversity, Program 7. Additional support from a CONICYT grant 79090016 is acknowledged. A.Z and R.D. are T.K, CONICYT—PhD fellows.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ledger, T., Zúñiga, A., Kraiser, T. et al. Aromatic compounds degradation plays a role in colonization of Arabidopsis thaliana and Acacia caven by Cupriavidus pinatubonensis JMP134. Antonie van Leeuwenhoek 101, 713–723 (2012). https://doi.org/10.1007/s10482-011-9685-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9685-8