Abstract
The exposure to environmental variations in pH and temperature has proven impacts on benthic ectotherms calcifiers, as evidenced by tradeoffs between physiological processes. However, how these stressors affect structure and functionality of mollusk shells has received less attention. Episodic events of upwelling of deep cold and low pH waters are well documented in eastern boundary systems and may be stressful to mollusks, impairing both physiological and biomechanical performance. These events are projected to become more intense, and extensive in time with ongoing global warming. In this study, we evaluate the independent and interactive effects of temperature and pH on the biomineral and biomechanical properties of Argopecten purpuratus scallop shells. Total organic matter in the shell mineral increased under reduced pH (~ 7.7) and control conditions (pH ~ 8.0). The periostracum layer coating the outer shell surface showed increased protein content under low pH conditions but decreasing sulfate and polysaccharides content. Reduced pH negatively impacts shell density and increases the disorder in the orientation of calcite crystals. At elevated temperatures (18 °C), shell microhardness increased. Other biomechanical properties were not affected by pH/temperature treatments. Thus, under a reduction of 0.3 pH units and low temperature, the response of A. purpuratus was a tradeoff among organic compounds (biopolymer plasticity), density, and crystal organization (mineral plasticity) to maintain shell biomechanical performance, while increased temperature ameliorated the impacts on shell hardness. Biopolymer plasticity was associated with ecophysiological performance, indicating that, under the influence of natural fluctuations in pH and temperature, energetic constraints might be critical in modulating the long-term sustainability of this compensatory mechanism.
Similar content being viewed by others
Introduction
Coastal areas experience large variations in temperature, salinity, oxygen and pH/pCO2 levels that have great influence on the performance of benthic organisms1,2,3. Off the Southern Pacific coast, regional (El Niño Southern Oscillation, ENSO) and global processes add up to seasonal, inter-annual and inter-decadal variability to the coastal ecosystems4,5. In addition, mesoscale processes such as coastal upwelling events show a wide range of variability in temperature, oxygen, and pH levels in very short time scales that have a great impact on animals inhabiting those ecosystems, especially calcifying invertebrates (i.e., mollusks, corals, barnacles, encrusting algae) which are vulnerable to these changes6,7,8,9. Coastal upwelling areas are characterized by seasonal, episodic events that bring subsurface waters of low temperature and low pH to shallower depths that may negatively impact benthic animals9,10,11,12. We now know that many organisms living in coastal upwelling areas either adapt to the local conditions7 or tolerate acute exposures to potentially stressful conditions11. Despite the low-temperature, low-pH upwelling conditions that generally persist in the coasts for periods of days, though more frequent and prolonged events due to increasing upwelling and favorable winds induced by global warming are expected for the following decades13,14, with potential impact on marine populations15.
The effects of temperature and pH/pCO2 on benthic organisms, mainly in the context of ocean warming and ocean acidification, have been extensively studied. Together they define key trade-offs between physiological processes, metabolic rates and shell calcification16,17, which also indicate the need for studies regarding the combined impacts of pH and temperature in the context of the whole organism18, and natural environmental variability7,10. However, the effects of environment-induced physiological compensations (due to changes in temperature and pH) on the shell structure and functionality of molluscan calcifiers (e.g., gastropods, bivalves) have been significantly less studied.
Marine molluscs have developed a broad diversity of shelled structures, the main function of which is to protect the organism against biological and environmental challenges (e.g., predators, waves forces). However, under high pCO2/low-pH conditions, the reduction of carbonate saturation state (Ω) favours the thermodynamic mineral solubility of calcium carbonate, thus increasing the cost of shell formation and calcification19. Nevertheless, several studies demonstrate that shell calcification can be maintained and even enhanced under reduced pH levels16,20,21,22,23, since marine calcifiers have a biological control over the calcification process24,25. Still, reduced seawater pH can affect shell properties, inducing mineral dissolution and changing the mineral organization and mechanical properties22,26,27,28,29,30.
Both changes in pH and temperature affect a suite of physiological processes (e.g., ingestion and metabolic rates), which also drives trade-offs that may alter shell calcification, structure, and functionality in mollusks. For instance, studies suggest that under low pH conditions, scallops maintain positive growth but at the cost of reducing the periostracum thickness and increasing the expression of functional molecules for biomineralization31, while gastropods show minor impacts on their metabolism, feeding, and shell growth rates, but trade-offs against changes in shell mineralogy22. Thus, mollusks may cope with low pH-induced dissolution by increasing protection with a thicker shell periostracum24,32,33, adjusting the amount and composition of organic material occluded within the shell mineral16,34,35, or altering shell mineral properties22,27,28,29,33. These studies suggest that shell properties may also be part of complex compensatory mechanisms to maintain overall performance and homeostasis when mollusks are exposed to low pH conditions. Increased temperatures can have similar impacts to low pH conditions in mollusk survival, growth, and development and temperature effects become exacerbated in combination with other concomitant stressors35. For instance, increasing temperature reduces shell integrity in M. edulis under limited food availability conditions, which may result from allocating energy from shell building to maintenance costs based on internal reserves36. However, in the context of combined impacts of temperature and pH, it is still poorly understood how biomineral and biomechanical properties of mollusk shells participate in these physiological compensations.
Argopecten purpuratus, is a native scallop species cultured in coastal embayment areas along the Southern Pacific coast of Peru and northern Chile, which are exposed to episodic events of coastal upwelling9,10,11,12. Empirical studies have suggested that winds inducing coastal upwelling could intensify at mid-latitudes in response to increased land-sea temperature differences driven by global warming37, while upwelling duration is also predicted to increase and lasting for several days14. This potential scenario could lead to scallop aquaculture sites being subjected to abnormally low temperature and pH conditions for periods longer than a week. Although, recent studies suggest that at high temperature (18 °C) and low pH (7.6), A. purpuratus show tradeoffs between positive growth and metabolism with biomineralization processes, which may result in local adaptation to natural low pH variability31, another study indicates that at low temperatures (14 °C), A. purpuratus showed increased shell dissolution and reduced growth rates when exposed simultaneously to low pH conditions (~ 7.7), which may have ramifications for the aquaculture of this species in the region30. Shell integrity and size are important ecological attributes with relevant implications in the thinning process performed by the aquaculture industry, when scallops are subjected to mechanical sieving, being segregated by size, with the objectives of reducing density, avoiding feeding interference, and thus reducing the time needed to reach market size30. Thus, in this study, we evaluated the effect of simultaneous changes in pH and temperature on juvenile A. purpuratus scallops, based on an orthogonal experimental design, incorporating the potential scenario of exposure to low temperature/low pH conditions over longer periods (> 2-week period), a potential future scenario in coastal upwelling areas at this latitude (Tongoy Bay, Northern Chile, 30° S). We specifically measured shell properties such as density, mineral composition, and organization, and associated biomechanical attributes.
Results
Environment, experimental scenarios, and seawater carbonate chemistry
Environmental variation of Tongoy Bay evidenced the influence of upwelling condition at Lengua de Vaca point (Fig. 1a). Elevated temperatures and pH values (17–18 °C and pH 8.0) were found in summer, whereas low temperature/low pH waters (14–15 °C and pH 7.70–7.75) were observed mostly during upwelling events in spring season (Fig. 1b). These natural fluctuations overlap perfectly with our selected “control scenario” (pH ~ 8.0/14 °C) and “upwelling condition” scenario, respectively. For the field sampling period, only one (1.9%; n = 36) of the pH observations recorded inside the bay falls below pH = 7.7 (Fig. 1b). Similarly, only one temperature measurement was above 18 °C (1.9%, n = 36). Thus, except for the experimental treatment of high temperature and low pH, the resulting experimental orthogonal combination of temperature and pH values (Table 1), represented extreme, but realistic environmental variations occurring in Tongoy Bay (Fig. 1b). Low values of carbonate saturation state occurred at low pH scenarios, but all treatments showed saturated conditions for calcite (Ω > 1, Table 1), the calcium carbonate mineral polymorph precipitated by Argopecten purpuratus.
Environmental context of the study. (a) Tongoy Bay and Pt. Lengua de Vaca (PLV), the nearby upwelling centre influencing fluctuations in temperature and pH inside the bay where scallops Argopecten purpuratus are farmed; (b) Variability in pH and temperature for the combination of experimental treatments (solid black squares); overlapped with the natural variability in temperature/pH (solid circles) recorded seasonally inside Tongoy Bay from dec-2014 to May-2016. A boxplot (median, mean and range) for all records of temperature and pH are shown at the top and right side of the graph, respectively.
Shell morphology and microstructure
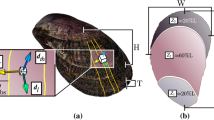
A. purpuratus shells have conspicuous ribs that radiate from the shell umbo. The outer shell surface is decorated by steps or terraces advancing parallel to the shell margin and that are associated to growth events. However, SEM and Micro-CT images showed that these structures are only observed in individuals raised at higher temperature conditions and control pH levels and absent under low temperature and low pH conditions (Fig. 2a). The shell mineral at the growing edge of the scallop shells is made of calcite crystal fibers of 1–2 microns thick, are arranged in parallel bundles, ending in well-defined rhombohedral faces with their elongation axis forming an angle to shell surface (Fig. 2b). Analysis of calcite crystal orientation by XRD confirm that crystals are highly aligned. The 006 pole figure displayed one maxima that is offset ca.30 degrees from the center (Fig. 2c), indicating that calcite crystals have their c-axis aligned but tilted 30 degrees from the shell toward the shell edge. In addition, the 104 pole figure showed three maxima around the position of the 006 maxima (i.e., the c-axis) that mark the disposition of {104} rhombohedral faces according to the three-fold symmetry of calcite (Fig. 2c). Thus, A. purpuratus shells have a fibrous microstructure of well-ordered calcite crystals with their three crystallographic axis co-oriented, though there is a significant scattering in the orientation of crystals (ca. 20–30 degrees), as indicated by the angular spread of maxima displayed in the pole figures or that measured in the 104 Gamma scans. When comparing the angular spread of calcite crystals from individuals grown at different conditions, the specimens at reduced pH conditions showed a significant increased angular spread (lower crystal orientation) (Fig. 2d). However, at higher temperature, there were no differences (Table 2) indicating that the effect of pH might be ameliorated at increased temperature (Fig. 2d).
Argopecten purpuratus shell surface morphology (micro-CT and SEM images) showing the ribs of scallops exposed to control and reduce pH conditions (a). SEM images of the shell microstructure in a transversal fracture, showing bundles of co-oriented calcite crystal fibers (b); The 006 and 104 pole figures (determined by 2D-XRD38,39) display the 3D orientation of the c-axis and rhombohedral faces of calcite crystals making the shell (c). Angular spread of the calcite crystal (determined from 104 Gamma scans), showing the scattering of the orientation of crystals in the shell outer surface (d).
Shell mineral and periostracum composition
The total and inter-crystalline organic matter (OM phase-1) content in the A. purpuratus shell mineral decreased significantly at warmer conditions, with no significant differences between pH levels (Fig. 3a,b), and both variables showed a significant antagonistic effect (Table 2). SEM observations showed that the shell periostracum was a thin fibrous organic layer coating the outer shell mineral surface, but that was irregularly distributed and even eroded under low pH conditions in both temperature treatments (Supplementary material, Figs. S1–S4). Analysis of the periostracum coating the outer shell surface by infrared spectroscopy showed an increase in the carbonate signal at reduced pH conditions in both temperature levels (Fig. 3c) indicating that the periostracum is thinner in these experimental treatments. Sulfates and polysaccharides decreased significantly at reduced pH levels and increased at higher temperatures (Fig. 3d–e), which in the case of polysaccharides lead to a significant and synergistic interactive effect between pH and temperature (Table 2). On the contrary, proteins showed a significant increase under low pH conditions, independent of temperature variations (Fig. 3f). Other bands associated with OH group and lipids on the shell periostracum of A. purpuratus showed no statistical differences among treatments (Table 2).
(a,b) Influence of temperature and pH on Argopecten purpuratus shell mineral composition (organic matter determined by TGA, % Mean ± SE); and (c–f) shell periostracum composition (determined by ATR-FTIR normalized signal, mean ± SE) Different letters represent significant differences among treatments using a post hoc Tukey HSD Test.
Shell density and biomechanical performance
Shell density showed a significant reduction at low pH conditions (F(1, 4) = 55.03, p = 0.002) with an effect reversal at warmer conditions (F(1, 4) = 21.03, p = 0.010), but the interaction between treatments was not significant. However, though overall higher shell density was recorded in animals exposed to increased temperatures shell density was reduced at the central and anterior (umbo) regions of the scallop shells (F(4, 48) = 3.83, p = 0.009; Fig. 4a–c). Microhardness of A. purpuratus shells increased under warmer conditions (F(6, 124) = 3.63; p = 0.002), with no effects of pH (Fig. 4d). In addition, strain or deformation of the shell material was affected by the interaction of shell condition (wet/dry) with orientation of compression test (Fig. 5a, F(2,86) = 4.18; p = 0.018). Wet shells and compression across the sagittal plane (shell thickness) showed increased strain respect to dry shells (F(1, 86) = 11.49, p = 0.001) and longitudinal axis (F(2, 86) = 34.14; p = 0.001). Mechanical stress was variable between shell condition and orientation (F(2, 86) = 4.93; p = 0.009), but evidencing a significant increase in stress along the longitudinal axis (F(2, 86) = 4.66; p = 0.012) (Fig. 5b). The elastic modulus (stiffness) showed a significant interaction between shell condition and orientation (F(2, 86) = 27.42; p < 0.001). Dry shells showed higher stiffness than wet shells (F(1, 86) = 41.23; p < 0.001), and mostly along the longitudinal axis (F(2, 86) = 144.42; p < 0.001); elevated temperature increased shell strain (F(1, 86) = 4.53; p = 0.036) and stiffness (F(1, 86) = 4.43; p = 0.038), but no effect of pH levels were observed (Fig. 5c).
Influence of temperature and pH on Argopecten purpuratus shell properties: (a–c) Shell density (mean ± SE) (determined by Micro-CT) at three different regions of the shell; (d) micro-hardness (determined by micro-indentation) at the shell growth edge. Different letters in (a) represent significant differences among treatments using a post hoc Tukey HSD. Posteriori test was not performed in (a–c,e) because the interaction of random with fixed effects.
Physiological rates and organic composition correlations
Physiological traits and shell dissolution previously recorded in A. purpuratus subjected to pH/temperature treatments (Table S1 in supplementary material, Lagos et al., 2016, Lardies et al., 2017) were correlated with variations in the organic content in the shell mineral and specific periostracum chemical components evaluated on the same scallop individuals (Fig. 6). Both total organic matter (Pearson–r = − 0.59, p = 0.004) and lipids (r = − 0.51, p = 0.01) were negatively correlated with metabolic rates recorded in the same scallops (Fig. 5a,b). On the contrary, ingestion rate was negatively correlated with shell sulfate content (r = − 0.38, p = 0.02), but positively correlated with the angular spread of calcite crystal (r = 0.33, p = 0.05) (Fig. 6c,d). Finally, the dissolution rate recorded in dead shell was positively correlated with the normalized signal of proteins remaining on the shell periostracum (r = 0.74, p = 0.02, Fig. 6e). However, these significant correlations between shell properties recorded in the same individual scallops must be interpreted with caution due to potential increased type 1 errors.
Significant correlations (r-Pearson) between (a) total organic matter in the carbonate matrix of Argopecten purpuratus; (b,c,e) organic compounds, and (d) calcite crystal angular spread recorded in the shell periostracum with metabolic(a,b), ingestion (c,d) and dissolution rates (e) (see also Table S1 supplementary material). Dots of different color indicate different combinations of pH/temperature treatments.
Discussion
Shell integrity is critical for the survival of marine calcifiers, and this study demonstrated that the scallop Argopecten purpuratus maintains shell biomechanical properties under low pH and low temperatures. Present results, along with previous studies done on the same individuals (see Table S1 in supplementary material), suggest that energetic demands of these physiological rates negatively impact the amount and composition of organic compounds deposited in the shell mineral matrix and surface periostracum of scallops. Our results reveal that organic content in the shell mineral and periostracum and calcite crystallographic orientation of A. purpuratus shells are plastic and correlated with variations in pH and temperature16,33. Low pH increased the amount of organic matter occluded within the shell mineral and altered the organic composition of the shell periostracum, increasing its protein content but reducing sulfates and polysaccharides. Regardless of temperature, low pH exposure increased the carbonate signal at the shell outer surface, which is indicative of a thinner periostracum16,31,34. A thinner periostracum may increase the exposure of the shell minerals, making them more susceptible to dissolution, affecting shell integrity and functionality. However, our biomechanical data (mechanical strain, stress, and elastic modulus) show that these environmentally induced changes in shell composition did not compromise shell biomechanical functionality, with exposure to high temperatures even improving shell density and microhardness.
Several studies have reported that under low pH conditions, the synthesis of biomineralization molecules (e.g., chitin) become stimulated, which may lead to increased shell growth31,40,41. However, other studies have reported decreased content of organic matter in mussel shells at low pH levels, but which is ameliorated under warmer conditions42,43. Our results show that at low pH and low temperature conditions, the periostracum protein content increases whereas sulfated polysaccharides content decreases. Similar results have been found in mussels exposed to natural low pH conditions16. However, in juvenile A. purpuratus scallops under stressful conditions of acidification (low pH) and low food supply, there are changes in the periostracum with an increase in polysaccharides production while proteins and lipids remain unaltered31. In addition, we found that the increase in proteins occur only under low pH and in combination with elevated temperature; conditions that are accompanied by a reduction in periostracum thickness as deduced by the increase in carbonates band intensity. In mussels, the carbonate band intensity is proportional to the periostracum thickness31,34. Thus, it may be suggested that in the case of A. purpuratus, the reduction in periostracum thickness results from a reduction in sulfated polysaccharides synthesis. In fact, shell dissolution of A. purpuratus increased significantly under low pH and increased temperatures conditions30 and in the present study we found that dissolution rate in dead shell was positively associated with protein content, suggesting that this might be an indirect effect of the reduced periostracum deposition. These results also suggest the protective role of sulfated polysaccharides component of the periostracum shell. Thus, under low pH and both temperature conditions, the response of A. purpuratus was to tradeoff the increase of proteins deposited in the periostracum to maintain positive shell growth, but at the cost of reducing sulfated polysaccharides production, which might compromise the protective role of periostracum against dissolution of shell carbonates. The expression of functional molecules associated with biomineralization such as chitin synthase may be underlying these changes in proteins content under low pH conditions31. This plasticity and trade-offs in organic compounds (i.e., biopolymers plasticity) of A. purpuratus scallop shells may represent an additional compensatory mechanism to confront environmental stress and maintain shell functionality in terms of structural support, protection and/or absorption of impact energy22,33
Low pH conditions also alter the shell density of A. purpuratus. Recent studies using micro-CT techniques demonstrate that gastropods exposed to low pH conditions can lose ca. 40% of the shell density46. Both shell density and porosity are relevant (but inverse) indicators of mechanical strength, which under low pH conditions, have shown variable impact on shell fragility45. In our study, we found ca. 20% reduction in shell density due to low pH conditions, and this reduction occurred in both newly deposited and non-growing shell regions. Previous studies highlighted that acidification, in addition to reducing shell precipitation, may increase dissolution at non-growing or older shell regions (e.g., apex or umbo)47. These changes suggest increased fragility of the whole shell. Thus, changes in shell density of A. purpuratus evidenced that low pH can increase the vulnerability to durophagous and bioeroders predators reducing survival opportunities in mollusks33,48. However, our results also show that increased temperatures ameliorate these impacts suggesting a relevant role of temperature conditions in maintaining shell density.
The influence of moderate warming (i.e., + 4 °C) ameliorates the impacts of low pH on molluscan physiology (e.g., survival, growth, and development)35. In addition, we found that the combined effect of acidification and higher temperature operated antagonistically changing the mineral organization (crystal orientation) of A. purpuratus shells and increasing crystal disorder, thereby increasing shell density. Similar results have been described in mollusks exposed to low pH conditions27,28,29, but these effects on biomineralization may disappear under dramatically increased temperatures44 (i.e., far over the 4 degrees used in this study) These alterations in crystal orientation, may allow more organic matter to precipitate, as observed under low pH conditions45. It has been suggested that low pH induces a progressive disorder in crystal orientation in mussels, which is inversely associated with the concentration of polysaccharides in their shell periostracum34. Thus, reduction in polysaccharides of the periostracum (and increased carbonate exposure), may affect the biomineralization and altering the crystal organization. Several studies report that the orientation of calcite crystals can be determined by an oriented nucleation on the organic matrix sheets that have molecular groups matching the disposition of ions (Ca2+) in the mineral surface (i.e., pseudo-epitaxis49,50), and the organic periostracum can also act as a substrate or template for oriented nucleation of calcite crystals51. Thus, changes in the crystal orientation of A. purpuratus shells suggest that some fundamental mechanisms of the mineralization may become impaired (i.e., epitaxial nucleation). These modifications in shell density and crystallography of A. purpuratus shells represent further evidence of mineral plasticity, suggested as a compensation mechanism to maintain biomechanical stability and functionality of mollusk shells22. This compensation mechanism is in agreement with our results, indicating no biomechanical weakness of A. purpuratus shells when exposed to the combined influence of low pH and temperature.
In general, it is suggested that the shell strength in scallops is a function of shell height, thickness, corrugation, and convexity of the whole shell52. In mollusks, experimental and natural low pH conditions alter shell size and thickness30,32,53, and several studies reported that low pH reduces the shell breaking strength in oysters54 and mussels40,55. Recent studies showed that gastropods produced tougher shells under low pH conditions, while the elastic modulus was maintained17 and that high temperature can ameliorate these impacts on shell mechanical properties54,55. Our results partially agree with these finding because A. purpuratus, independent of pH conditions, produce a tougher shell at increased temperatures while the elastic module, strain and stress remained unaltered. In addition, the organic matter provided successful elasticity and deformation45, despite the low proportion in the scallop shells (~ 1%), which, jointly with trade-offs between proteins and polysaccharides/lipids, could be responsible for maintaining shell mechanical strength. Nevertheless, biomechanical test evaluation proved to be quite complex and shows the strong influence of shell conditions (wet/dry) and the direction of the compression tests, which might prevent determination of differences in these shell biomechanical properties between experimental groups56. Thus, biomechanical responses of mollusk shell may be highly variable, from negative effects on the shell strength31,54,55,57 to non-effects in gastropods17,22 and scallops (this study). Our results also indicate that variations in biomechanical responses of A. purpuratus shell could arise from variations in the biomaterial properties used to build different structural parts of the shell and orientation of measurements (i.e., anisotropy). For instance, shell stiffness increased along the shell ribs, which is also parallel to the growth of calcite crystals. Whether these variations depend on the hierarchical structure of the shell (i.e., micro-, nano-scales45), the building materials, shell geometry, shell density or others whole-shell properties, deserves more comparative studies specially to understand the interaction of climate stressors, shell structural changes and how these affect their biomechanical properties.
Finally, metabolic and feeding performance of A. purpuratus scallops is elevated at increased temperature11 and these traits were negatively associated with alteration in organic matter of the shell mineral organic matrix, periostracum chemical composition and shell mineral organization (calcite crystal orientation). These relationships show how physiological processes associated with energy acquisition may interplay with process modulating shell biomineralization. Additionally, associations with physiological traits evidenced that the structural and mineral components and biomechanical performance of scallop shells are part of the overall compensations occurring when mollusks confront stress conditions imposed by low pH and temperature fluctuations. Environmentally-induced changes in shell and periostracum organic composition (i.e., biopolymers plasticity), shell density and mineral organization (i.e., mineral plasticity22) seem the most plausible mechanisms used by A. purpuratus to maintain a functional shell and therefore confront environmental and biological threats. However, the lack of effect of elevated temperature on shell resistance and its positive effect on scallop shell hardness must be interpreted with caution, since elevated temperature (18 °C) conditions are extreme events, rarely occurring inside Tongoy Bay. Future predictions suggest an increase in upwelling intensity and duration at mid and high latitudes14. Although, the driving mechanisms are not clear, coastal upwelling in eastern boundary current systems have intensified and it seems that the increasing trend will continue13,37. Thus, persistent upwelling-induced low temperature and low pH conditions represent a potential threat for the structural composition and mineral density of scallop shells, imposing restrictions on the physiological performance of the whole organism in field conditions12. Further studies are required to establish if this biopolymer and mineral plasticity in A. purpuratus shells can be sustained over ecologically relevant time (months-years) and under the persistent influence of coastal upwelling. Our results highlight the crucial importance of monitoring upwelling-associated carbonate systems parameters in the aquaculture industry12,58 and the potential impacts of carbonate chemistry on shell strength of both wild populations and farmed scallops inside Tongoy Bay. Considering the high variability in the production of this valuable resource30, these results could contribute to the better management and adaptive capacity of this aquaculture activity.
Methods
Environment, experimental scenarios, and seawater carbonate chemistry
Juvenile Argopecten purpuratus scallops (~ 41 mm ± 1.3 SD shell length) were collected from Invertec—Ostimar Co. scallop farm located in Tongoy Bay (30° S, central Chile, Fig. 1a). Scallops were transported in a thermobox (ca. 14 °C) to the Calfuco Coastal Laboratory (Valdivia, Chile), kept for acclimation during 3 days under running seawater (13−14 °C), natural photoperiod, and fed daily with microalgae (Tetraselmis spp., ~ 65 × 106 cells ml−1). To examine the combined effects of temperature and pH, an orthogonal experimental design was considered by incorporating four experimental treatments, where three of these treatments were based on natural environmental conditions observed in Tongoy Bay: (1) Control condition (non-upwelling): 14 °C/pH ~ 8.0; (2) Low temperature/low pH (upwelling condition) (14 °C/pH ~ 7.7); (3) High temperature/high pH (summer condition) (18 °C/ pH ~ 8.0) and (4) an artificial scenario of high temperature/low pH condition (18 °C/pH ~ 7.7), which is not possible to observe at the temporal scale of the present study (upwelling cycle of days/week), but possible upon long-term climatic projections for this region59. Each treatment was replicated five times (5 aquaria) and each replicate contained four scallops. Bee tags were used to identify and track individual scallops during the experiment. All these experimental processes and animal manipulations were performed in accordance with guidelines and regulations established by the Chilean law.
We used a semi-automatic CO2 equilibration system to obtain selected low pH scenarios. Blended dry air was generated by compressing atmospheric air (117 psi, oil-free compressor) with pure CO2 using mass flow controllers (MFCs, AALBORG); this blend was then bubbled into experimental aquaria (replicates) and head tanks reaching pCO2 ~ 900 μatm in seawater60. Briefly, the pCO2-induced acidification ranges from 367 to 435 μatm for the control scenario, and 891–969 μatm according A2 emission scenario for the year 210061, which yield nominal pH–levels of 8.0 and 7.7 units. These two pH treatments were combined in a full factorial experimental design with two temperature treatments (14 °C and 18 °C). The scallops were exposed for 18 d, since one of the main focuses of our study was determine how scallops could respond to prolonged low temperature/low pH conditions, upon a scenario of extended exposure to upwelling condition, which typically last from 2 up to 8 days at present12,62. Over the experimental period, temperature, salinity, pHNBS and total alkalinity (AT) were monitored every 3 days to estimate the rest of carbonate system parameters (i.e., pCO2, calcite saturation state Ω) inside the experimental aquaria. Additionally, in field conditions we monitored seasonal variability of carbonate system variations inside Tongoy bay from December 2014 to May 2016. Discrete shipboard sampling of temperature was conducted at 2 and 8 m depth, using a SeaBird SBE-19plus CTD profiler. Additionally, discrete water samples for pH estimates were collected and measurements were standardized to total scale (pHT) by using a METROHM 713 pH meter (input resistance > 1013 Ohm, 0.1 mV sensitivity, and nominal resolution 0.001 pH units) using a glass combined double junction Ag/AgCl electrode (METHROM model 6.0219.100) calibrated with 8.089 Tris buffer 25 °C following DOE potentiometric method10,60.
Shell morphology and microstructure
Shell pieces samples fractured from the growing shell edge were cleaned with deionized water and then carbon-coated (HITACHI UHS evaporator) prior to observations using Auriga CrossBeam Workstation operated at 5 kV (ZEISS, SEM). Crystallographic orientation was measured in fragments collected from the shell edge (ca. 3 × 3 mm, n = 5 per treatment, 1 per aquaria) using X-ray single-crystal diffractometer equipped with a CCD area detector (D8 SMART APEX, BRUKER, Germany). Working conditions of diffraction measurements were Mo Ka (0.7093 Å), 50 kV and 30 mA, a pin-hole collimator of 0.5 mm in diameter, and an exposure time of 20 s per frame. To quantify the orientation of crystals from 2D-XRD patterns or frames, the width of the peaks displayed in the intensity profile along 104 rings of individual frames (104 Gamma scan), which represent the angular spread or scattering in the tilting of the c–axis of calcite crystal within the shell mineral, was measured34. In selected samples, a set of 2D-XRD patterns (frames) was registered while rotating the sample around Φ angle (every 5 degrees). Pole figures displaying the three-dimensional distribution of specific crystallographic directions of calcite crystals (i.e., 006, 104,) were determined using XRD2DScan software v.7.038,39.
Shell mineral and periostracum composition
After the experiment, shells were washed with deionized water, oven dried at 60 °C for 4 h and small pieces (ca. 2 × 2 mm) were cut from the growing shell edge. A. purpuratus shell organic content was analyzed by Thermo-Gravimetric Analysis (TGA, METTLER-TOLEDO DSC1, Zurich, Switzerland). Pieces of the growing edge (n = 5 per treatment, 1 per aquaria), were powdered and ca. 25 mg was used for the analysis (25–900 °C; 20 °C min-1 heating rate). Main weight loss events occurred in the range of 20–180 °C due to loss of residual water; then to the combustion of inter– (ca. 180–400 °C; OM phase-1) and intra– (400–600 °C; OM phase-2) mineral organic matter (OM), and finally at temperatures > 600 °C to the CO2 loss due to thermal decomposition of carbonates31,34. Shell periostracum chemical composition was determined by infrared spectroscopy using Fourier Transformed Infrared Spectrometer (FTIR) with Attenuated Total Reflection (ATR) unit (FTIR model 6600, ATR Pro, JASCO, Japan) as described in more detail elsewhere31. Briefly, the outer surface of shell samples (n = 5 per treatment) randomly collected from the growing margin were pressed against the ATR window, and reflectance spectra were recorded at a 2 cm−1 resolution for 50 scans. The relative amounts of water, proteins, sulfates, carbonates, polysaccharides, and lipids, among others, were estimated from the absorption peak areas associated to each chemical component (e.g., O–H: water; C–H: lipids or fatty acids; amide bond: proteins; C–O: carbonates; S–O: sulfates; COC: sugars/polysaccharides), and then normalized to the total area of the FTIR spectra to provide a semiquantitative analysis.
Shell density and biomechanical performance
Shell density (g/cm3) was evaluated (n = 5 per treatment) using Micro-Computed Tomography scanning (Micro-CT Skyscan 1278, BRUKER, Belgium). We use 300 two-dimensional scans per sample, and then convert into a 3D reconstructed model (CT-Vol software v. 2.3.2.0; https://www.bruker.com/en/products-and-solutions/microscopes/3d-x-ray-microscopes.html). Measurements were standardized using phantoms (i.e., 0.25 y 0.75 g/cm3) provided for bone density (CT-Analyzer software v1.13; https://www.bruker.com/en/products-and-solutions/microscopes/3d-x-ray-microscopes.html)50,63. Density measurements were randomly performed in 3 regions of the shell (a: newly growing edge; b: center; and c: umbo), representing a 3D volume of ca. 25µm3 per side. At the growing edge (ca. 1 × 1 mm), shell microhardness was measured using a Zwick hardness tester with a Vickers indenter (ASTM C1327-15). Shell samples were placed in 30 mm silicon molds, embedded in epoxy resin (12 h), then ground with silicon carbide paper (from 600 to 2400 grids) and polished with aluminum oxide suspensions (from 0.1 to 0.04 um). Vickers microhardness tests (8–9 random indentations, n = 4 shells per treatments, 1 per aquaria) were conducted at room temperature, applying a load of 2.94 N and dwell time of 10 s17,22. 400 × photographs were used to measure the diagonal of the imprint indented area using ImageJ software v.152a (https://imagej.nih.gov/ij/)64. Then, the Vickers Hardness (MPa) was calculated as HV = 0.1891 P/d2 where P is the applied load (N) and d the mean of the two measured diagonals (mm). Uniaxial compression tests were performed using a universal texturing machine (Instron 3342). The compression test uses shell fragment (2 × 2 mm) from the growing shell edge and oriented along three main planes of the shell fragments: longitudinal to the shell ribs; across the sagittal section (thickness) and transversal to the ribs. Tensile tests were also performed under dry and re-hydration conditions56, using synthetic seawater (INSTANT OCEAN) at 33 psu in salinity.
Statistical analyses
In all cases shell properties measurements were performed on individual shells to avoid pseudo replication issues. Repeated measurements were done on scallop shells subjected to TGA, ATR, XRD analyses but analyzed separately using factorial ANOVAs including Tukey’s HSD as a posteriori test to evaluate the effects of pH and temperature treatments on organic matter in the shell carbonate matrix and organic content in shell periostracum. Differences in mineral density and micro-hardness were tested using a mixed factorial nested ANOVA, regarding shell areas (edge, center, and umbo within the same shell) and repeated indentations within shell as random effects, and temperature—pH treatments as fixed effects. Finally, using r–Pearson correlation index, we explored the paired relationship between organic matter and compounds recorded in the shell matrix and periostracum with physiological rates for exactly the same individual scallops11,30. ANOVAs and correlations were carried out using MINITAB v14 (https://www.minitab.com).
Data availability
Datasets generated and analyzed during the current study are available from the corresponding author.
References
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D. & Hales, B. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320, 1490–1492 (2008).
Hofmann, G. E. et al. High-frequency dynamics of ocean ph: A multi-ecosystem comparison. PLoS ONE 6(12), e28983 (2011).
Kroeker, K. J. et al. Interacting environmental mosaics drive geographic variation in mussel performance and predation vulnerability. Ecol. Lett. 19, 771–779 (2016).
Gutiérrez, D. et al. Coastal cooling and increased productivity in the main upwelling zone off Peru since the mid-twentieth century. Geophys. Res. Lett. 38, L07603. https://doi.org/10.1029/2010GL046324 (2011).
Aiken, C. M., Navarrete, S. A. & Pelegrí, J. L. Potential changes in larval dispersal and alongshore connectivity on the central Chilean coast due to an altered wind climate. J. Geophys. Res. 116, G04026. https://doi.org/10.1029/2011JG001731 (2011).
Lagos, N. A., Castilla, J. C. & Broitman, B. Spatial Environmental correlates of intertidal recruitment: A test using barnacles in northern Chile. Ecol. Monogr. 78, 245–261 (2008).
Vargas, C. A. et al. Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 1, 84. https://doi.org/10.1038/s41559-017-0084 (2017).
Broitman, B. R. et al. Phenotypic plasticity is not a cline: Thermal physiology of an intertidal barnacle over 20° of latitude. J. Anim. Ecol. 00, 1–12. https://doi.org/10.1111/1365-2656.13514 (2021).
Ramajo, L. et al. Physiological responses of juvenile Chilean scallops (Argopecten purpuratus) to isolated and combined environmental drivers of coastal upwelling. ICES J. Mar. Sci. 76, 1836e1849 (2019).
Saavedra, L. M., Saldías, G., Broitman, B. & Vargas, C. Carbonate chemistry dynamics in shellfish farming areas along the Chilean coast: Natural ranges and biological implications. ICES J. Mar. Sci. 78, 323–339 (2021).
Lardies, M. A. et al. Physiological and histopathological impacts of increased carbon dioxide and temperature on the scallops Argopecten purpuratus cultured under upwelling influences in northern Chile. Aquaculture 479, 455–466 (2017).
Ramajo, L. et al. Upwelling intensity modulates the fitness and physiological performance of coastal species: Implications for the aquaculture of the scallop Argopecten purpuratus in the Humboldt Current System. Sci. Total Environ. 745, 140949 (2020).
Bakun, A. Global climate change and intensification of coastal ocean upwelling. Science 247, 198–201 (1990).
Wang, D. et al. Intensification and spatial homogenization of coastal upwelling under climate change. Nature 518, 390–394 (2015).
Kim, T. W., Barry, J. P. & Micheli, F. The effects of intermittent exposure to low-pH and low-oxygen conditions on survival and growth of juvenile red abalone. Biogeosciences 10, 7255–7262 (2013).
Ramajo, L. et al. Plasticity and trade-offs in physiological traits of intertidal mussels subjected to freshwater-induced environmental variation. Mar. Ecol. Prog. Ser. 553, 93–109 (2016).
Leung, J. Y., Connell, S. D., Nagelkerken, I. & Russell, B. D. Impacts of near-future ocean acidification and warming on the shell mechanical and geochemical properties of gastropods from intertidal to subtidal zones. Environ. Sci. Technol. 51, 12097–12103 (2017).
Findlay, H. et al. Calcification, a physiological process to be considered in the context of the whole organism. Biogeosciences Discuss. 6, 2267–2284 (2009).
Waldbusser, G. et al. Saturation-state sensitivity of marine bivalves larvae to ocean acidification. Nat. Clim. Change 5, 273–280 (2015).
Tunnicliffe, V. et al. Survival of mussels in extremely acidic waters on a submarine volcano. Nat. Geosci. 2, 344–348 (2009).
Ries, J. B., Cohen, A. L. & McCorkle, D. C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134 (2009).
Leung, J. Y., Russell, B. D. & Connell, S. D. Mineralogical plasticity acts as a compensatory mechanism to the impacts of ocean acidification. Environ. Sci. Technol. 51, 2652–2659 (2017).
Duarte, C. et al. The energetic physiology of juvenile mussels, Mytilus chilensis (Hupe): The prevalent role of salinity under current and predicted pCO2 scenarios. Environ. Pollut. 242, 156–163 (2018).
Rodolfo-Metalpa, R. et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change. 1, 308–312 (2011).
Waldbusser, G. et al. Slow shell building, a possible trait for resistance to the effects of acute ocean acidification. Limnol. Oceanogr. 61, 1969–1983 (2016).
Fitzer, S. C. et al. Ocean acidification and temperature increase impact mussel shell shape and thickness: Problematic for protection?. Ecol. Evol. 5, 4875–4884 (2015).
Fitzer, S. C., Phoenix, V. R., Cusack, M. & Kamenos, N. A. Ocean acidification impacts mussel control on biomineralization. Sci. Rep. 28, 6218 (2014).
Fitzer, S. C., Cusack, M., Phoenix, V. R. & Kamenos, N. A. Ocean acidification reduces the crystallographic control in juvenile mussel shells. J. Struct. Biol. 188, 39–45 (2014).
Fitzer, S. C. et al. Biomineral shell formation under ocean acidification: A shift from order to chaos. Sci. Rep. 6, 21076 (2016).
Lagos, N. A. et al. Effects of temperature and ocean acidification on shell characteristics of Argopecten purpuratus: Implications for scallop aquaculture in an upwelling-influenced area. Aquac. Environ. Interact. 8, 357–370 (2016).
Ramajo, L. et al. Biomineralization changes with food supply confer juvenile scallops (Argopecten purpuratus) resistance to ocean acidification. Glob. Chang. Biol. 22, 2025–2203 (2016).
Osores, S. J. et al. Plasticity and inter-population variability in physiological and life-history traits of the mussel Mytilus chilensis: A reciprocal transplant experiment. J. Exp. Mar. Biol. Ecol. 490, 1–12 (2017).
Telesca, L. et al. Plasticity and environmental heterogeneity predict geographic resilience patterns of foundation species to future change. Glob. Chang. Biol. https://doi.org/10.1111/gcb.14758 (2020).
Grenier, C. et al. The combined effects of salinity and pH on shell biomineralization of the edible mussel Mytilus chilensis. Environ. Pollut. 263, 114555 (2020).
Kroeker, K. J. et al. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (2013).
Mackenzie, C. L. et al. Ocean warming, more than acidification, reduces shell strength in a commercial shellfish species during food limitation. PLoS ONE 9(1), e86764 (2014).
Rykaczewski, R. R. et al. Poleward displacement of coastal upwelling-favorable winds in the ocean’s eastern boundary currents through the 21st century. Geophys. Res. Lett. 42, 6424–6431 (2015).
Rodríguez-Navarro, A. B. Rapid quantification of avian eggshell microstructure and crystallographic-texture using two-dimensional X-ray diffraction. Br. Poult. Sci. 48, 133–144 (2007).
Rodríguez-Navarro, A. B. XRD2DScan: New software for polycrystalline materials characterization using two-dimensional X-ray diffraction. J. Appl. Cryst. 39, 905–909 (2006).
Li, S. et al. Interactive effects of seawater acidification and elevated temperature on biomineralization and amino acid metabolism in the mussel Mytilus edulis. J. Exp. Biol. 218, 3623–3631 (2015).
Li, S. et al. Interactive effects of seawater acidification and elevated temperature on the transcriptome and biomineralization in the pearl oyster Pinctada fucata. Environ. Sci. Technol. 50, 1157–1165 (2016).
Gestoso, I., Arenas, F. & Olabarria, C. Ecological interactions modulate responses of two intertidal mussel species to changes in temperature and pH. J. Exp. Mar. Biol. 474, 116–125 (2016).
Babarro, J. M., Abad, M. J., Gestoso, I., Silva, E. & Olabarria, C. Susceptibility of two co-existing mytilid species to simulated predation under projected climate change conditions. Hydrobiologia 807, 247–261 (2018).
Barthelat, F., Rim, J. E. & Espinosa, H. D. A review on the structure and mechanical properties of mollusk shells: Perspectives on synthetic biomimetic materials. In Applied Scanning Probe Methods XIII (eds Bhushan, B. & Fuchs, H.) 17–44 (Springer, 2009).
Leung, J. Y. et al. Calcifiers can adjust shell building at the nanoscale to resist ocean acidification. Small 16, 2003186 (2020).
Chatzinikolaou, E., Grigoriou, P., Keklikoglou, K., Faulwetter, S. & Papageorgiou, N. The combined effects of reduced pH and elevated temperature on the shell density of two gastropod species measured using micro-CT imaging. ICES J. Mar. Sci. 74, 1135–1149 (2017).
Nienhuis, S., Palmer, R. & Harley, C. Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail. Proc. R. Soc. Lond. B Biol. Sci. 277, 2553–2558 (2010).
Bourdeau, P. E. Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90, 1659–1669 (2009).
Weiner, S. & Addadi, L. Crystallization pathways in biomineralization. Annu. Rev. Mater. Sci. 41, 21–40 (2011).
Nudelman, F. Nacre biomineralisation: A review on the mechanisms of crystal nucleation (In Seminars in cell & developmental biology), 2–10 (Academic Press, 2015).
Harper, E. M., Checa, A. G. & Rodríguez-Navarro, A. B. Organization and mode of secretion of the granular prismatic microstructure of Entodesma navicular (Bivalvia: Mollusca). Acta Zool. 90, 132e141 (2009).
Pennington, B. J. & Currey, J. D. A mathematical model for the mechanical properties of scallop shells. J. Zool. 202, 239–263 (1984).
Yevenes, M. A., Lagos, N. A., Farías, L. & Vargas, C. A. Greenhouse gases, nutrients and the carbonate system in the Reloncaví Fjord (Northern Chilean Patagonia): Implications on aquaculture of the mussel, Mytilus chilensis, during an episodic volcanic eruption. Sci. Total Environ. 669, 49–61 (2019).
Dickinson, G. H. et al. Interactive effects of salinity and elevated CO2 levels on juvenile eastern oysters, Crassostrea virginica. J. Exp. Biol. 215, 29–43 (2012).
Gaylord, B. et al. Functional impacts of ocean acidification in an ecologically critical foundation species. J. Exp. Biol. 214, 2586–2594 (2011).
O’Toole-Howes, M. et al. Deconvolution of the elastic properties of bivalve shell nanocomposites from direct measurement and finite element analysis. J. Mater. Res. 34, 2869–2880 (2019).
Auzoux-Bordenave, S. et al. Ocean acidification impacts growth and shell mineralization in juvenile abalone (Haliotis tuberculata). Mar. Biol. 167, 11 (2020).
Torres, R. et al. Evaluation of a semiautomatic system for long-term seawater carbonate chemistry manipulation. Rev. Chil. Hist. Nat. 86, 443–451 (2013).
IPCC. Climate Change 2021. The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Eds. Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou). Cambridge University Press. In Press. (2021).
DOE. Handbook of methods for the analysis of the various parameters of the carbon dioxide system in seawater; version 2 (eds. Dickson, A.G. & Goyet, C.), (ORNL/CDIAC, 74, 1994).
Meinshausen, M. et al. The RPC greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Change. 109, 213–241 (2011).
Rahn, D. A., Rosenblüth, B. & Rutllant, J. A. Detecting subtle seasonal transitions of upwelling in North-Central Chile. J. Phys. Oceanogr. 45, 854–867 (2015).
Meng, Y., Guo, Z., Yao, H., Yeung, K. W. & Thiyagarajan, V. Calcium carbonate unit realignment under acidification: A potential compensatory mechanism in an edible estuarine oyster. Mar. Pollut. Bull. 139, 141–149 (2019).
Rasband, W. S. ImageJ U.S. National Institute of Health, Maryland, USA (1997–2020).
Acknowledgements
This study was funded by PIA ANID ACT 172037 for international collaborative research among Chile (NAL, MAL, JV, CG-H, CD) and Spain (ARN, CG). Author also acknowledges support from Fondecyt 1190444 (MAL), Fondecyt 1210171 (CAV) and ANID – Millennium Science Initiative Program – ICN2019_015 (SECOS) (NAL, MAL and CAV). ANID doctoral scholarhip #21210012 to SB. ANID PFCHA / Doctorados Becas Chile Chile/2019-CEL00011051 to AA-O and DICYT from USACH to AA-O and CG-H during the execution of sample processing and analysis. We also acknowledge to Anita Quiroga, Jhonny Rojas for their support in lab procedures, and the staff at BIO-CT Lab (Universidad de Chile). We are grateful to B. Broitman and two anonymous reviewers who provided valuable comments on previous version of the manuscript.
Author information
Authors and Affiliations
Contributions
N.A.L. conceived and designed the study, acquired, and analysed data, wrote the paper. S.B., M.A.L., C.A.V. and C.D. conceived and designed the experiment, acquired and analysed data, and revised the paper, A.R.-N. and C.G. acquired and analysed shell organic composition, crystal orientation data, and revised the paper; A.A.-O. and C.G.-H. acquired and analysed compression tests and mineral density data, revised the paper. I.B. and J.F.V. acquired and analysed microhardness data, revised the paper. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lagos, N.A., Benítez, S., Grenier, C. et al. Plasticity in organic composition maintains biomechanical performance in shells of juvenile scallops exposed to altered temperature and pH conditions. Sci Rep 11, 24201 (2021). https://doi.org/10.1038/s41598-021-03532-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03532-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.