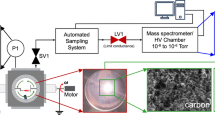
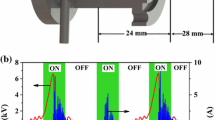
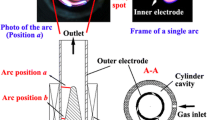
Abstract
We studied hydrogen sulfide (H2S) decomposition into hydrogen (H2) and sulfur (S2) in a gliding arc plasmatron (GAP) and microwave (MW) plasma by a combination of 0D and 2D models. The conversion, energy efficiency, and plasma distribution are examined for different discharge conditions, and validated with available experiments from literature. Furthermore, a comparison is made between GAP and MW plasma. The GAP operates at atmospheric pressure, while the MW plasma experiments to which comparison is made were performed at reduced pressure. Indeed, the MW discharge region becomes very much contracted near atmospheric pressure, at the conditions under study, as revealed by our 2D model. The models predict that thermal reactions play the most important role in H2S decomposition in both plasma types. The GAP has a higher energy efficiency but lower conversion than the MW plasma at their typical conditions. When compared at the same conversion, the GAP exhibits a higher energy efficiency and lower energy cost than the MW plasma.











Similar content being viewed by others
References
Eow JS (2002) Recovery of sulfur from sour acid gas: a review of the technology. Environ Prog 21:143–162
Fridman A (2008) Plasma chemistry. Cambridge University Press, Cambridge
Kim H-H (2004) Nonthermal plasma processing for air-pollution control: a historical review, current issues, and future prospects. Plasma Process Polym 1:91–110
Snoeckx R, Bogaerts A (2017) Plasma technology: a novel solution for CO2 conversion? Chem Soc Rev 46:5805–5863
Farrell A, Keith DW, Jensen MW, Ross M (2001) Hydrogen as a transportation fuel. Environ Sci Policy Sustain Dev 43:43–45
Traus I, Suhr H (1992) Hydrogen sulfide dissociation in ozonizer discharges and operation of ozonizers at elevated temperatures. Plasma Chem Plasma Process 12:275–285
Traus I, Suhr H, Harry JE, Evans DR (1993) Application of a rotating high-pressure glow discharge for the dissociation of hydrogen sulfide. Plasma Chem Plasma Process 13:77–91
Helfritch DJ (1993) Pulsed corona discharge for hydrogen sulfide decomposition. IEEE Trans Ind Appl 29:882–886
Nicholas JE, Amodio CA, Baker MJ (1979) Kinetics and mechanism of the decomposition of H2S, CH3SH and (CH3)2S in a radio-frequency pulse discharge. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 75:1868–1875
Zhao GB, John S, Zhang JJ, Hamann JC, Muknahallipatna SS, Legowski S, Ackerman JF, Argyle MD (2007) Production of hydrogen and sulfur from hydrogen sulfide in a nonthermal-plasma pulsed corona discharge reactor. Chem Eng Sci 62:2216–2227
Nunnally TP (2011) Application of low current gliding arc plasma discharges for hydrogen sulfide decomposition and carbon dioxide emission reduction, PhD dissertation, Drexel University
Godefroid T (2016) Traitement de mélange H2S par plasma micro-onde. Bilan de matière et énergétique (Mons)
Luinstra EA, Energy AA, Research S (1995) Hydrogen from H2S: technologies and economics. Sulfotech Research, Calgary
Balebanov AV, Butylin BA, Zhivotov VKKV, Matolich RMMS (1985) Dissociation of hydrogen sulfide in a plasma. Dokludy Phys Chem 283:709
Gutsol A, Rabinovich A, Fridman A (2011) Combustion-assisted plasma in fuel conversion. J Phys D Appl Phys 44:274001
Sassi M, Amira N (2012) Chemical reactor network modeling of a microwave plasma thermal decomposition of H2S into hydrogen and sulfur. Int J Hydrogen Energy 37:10010–10019
Kaloidas V, Papayannakos N (1989) Kinetics of thermal, non-catalytic decomposition of hydrogen sulphide. Chem Eng Sci 44:2493–2500
Dowling NI, Clark PD (1999) Kinetic modeling of the reaction between hydrogen and sulfur and opposing H2S decomposition at high temperatures. Ind Eng Chem Res 38:1369–1375
Pancheshnyi S, Eismann B, Hagelaar GJM, Pitchford LC (2008) Computer code ZDPlasKin. University of Toulouse, LAPLACE, CNRS-UPS-INP, Toulouse, France
Hagelaar GJM, Pitchford LC (2005) Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci Technol 14:722
Rawat P, Iga I, Lee M-T, Brescansin LM, Homem MGP, Machado LE (2003) Cross sections for elastic electron–hydrogen sulfide collisions in the low-and intermediate-energy range. Phys Rev A 68:52711
Wang W, Murphy AB, Rong M, Looe HM, Spencer JW (2013) Investigation on critical breakdown electric field of hot sulfur hexafluoride/carbon tetrafluoride mixtures for high voltage circuit breaker applications. J Appl Phys 114:103301
Wang W, Snoeckx R, Zhang X, Cha MS, Bogaerts A (2018) modeling plasma-based CO2 and CH4 conversion in mixtures with N2, O2, and H2O: the bigger plasma chemistry picture. J Phys Chem C 122:8704–8723
Aerts R, Somers W, Bogaerts A (2015) Carbon dioxide splitting in a dielectric barrier discharge plasma: a combined experimental and computational study. Chemsuschem 8:702–716
Snoeckx R, Aerts R, Tu X, Bogaerts A (2013) Plasma-based dry reforming: a computational study ranging from the nanoseconds to seconds time scale. J Phys Chem C 117:4957–4970
Bogaerts A, Wang W, Berthelot A, Guerra V (2016) Modeling plasma-based CO2 conversion: crucial role of the dissociation cross section. Plasma Sources Sci Technol 25:55016
Ferziger JH, Peric M (2012) Computational methods for fluid dynamics. Springer, Berlin
Wilcox DC et al (1998) Turbulence modeling for CFD, vol 2. DCW Industries, La Canada
Kolev S, Bogaerts A (2015) A 2D model for a gliding arc discharge. Plasma Sources Sci Technol 24:15025
Ramakers M, Trenchev G, Heijkers S, Wang W, Bogaerts A (2017) Gliding arc plasmatron: providing an alternative method for carbon dioxide conversion. Chemsuschem 10:2642–2652
Georgieva V, Berthelot A, Silva T, Kolev S, Graef W, Britun N, Chen G, van der Mullen J, Godfroid T, Mihailova D et al (2017) Understanding microwave surface-wave sustained plasmas at intermediate pressure by 2D modeling and experiments. Plasma Process Polym 14:1600185
Trenchev G, Kolev S, Wang W, Ramakers M, Bogaerts A (2017) CO2 Conversion in a gliding arc plasmatron: multidimensional modeling for improved efficiency. J Phys Chem C 121:24470–24479
Bongers W, Bouwmeester H, Wolf B, Peeters F, Welzel S, van den Bekerom D, den Harder N, Goede A, Graswinckel M, Groen PW, Kopecki J, Leins M, van Rooij G, Schulz A, Walker M, van de Sanden R (2017) Plasma-driven dissociation of CO2 for fuel synthesis. Plasma Process Polym 14:1600126
Sendt K, Jazbec M, Haynes BS (2002) Chemical kinetic modeling of the H/S system: H2S thermolysis and H2 sulfidation. Proc Combust Inst 29:2439–2446
Tesner PA, Nemirovskii MS, Motyl DN (1991) Kinetics of the thermal decomposition of hydrogen sulfide at 600–1200 °C. Kinet Catal 31:1081–1083
Woiki D, Roth P (1994) Kinetics of the high-temperature H2S decomposition. J Phys Chem 98:12958–12963
Peng J, Hu X, Marshall P (1999) Experimental and ab initio investigations of the kinetics of the reaction of H atoms with H2S. J Phys Chem A 103:5307–5311
Schofield K (1973) Evaluated chemical kinetic rate constants for various gas phase reactions. J Phys Chem Ref Data 2:25–84
Higashihara T, Saito K, Murakami I (1980) The dissociation rate of S2 produced from COS pyrolysis. Bull Chem Soc Jpn 53:15–18
Tiee JJ, Wampler FB, Oldenborg RC, Rice WW (1981) Spectroscopy and reaction kinetics of HS radicals. Chem Phys Lett 82:80–84
Shiina H, Oya M, Yamashita K, Miyoshi A, Matsui H (1996) Kinetic studies on the pyrolysis of H2S. J Phys Chem 100:2136–2140
Acknowledgments
This work was supported by the Scientific Research Foundation from Dalian University of Technology, DUT19RC(3)045. We gratefully acknowledge T. Godfroid (Materia Nova) for sharing the experimental data about the MW plasma. The calculations were performed using the Turing HPC infrastructure at the CalcUA core facility of the Universiteit Antwerpen (UAntwerpen), a division of the Flemish Supercomputer Center VSC, funded by the Hercules Foundation, the Flemish Government (department EWI) and the UAntwerpen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Figs. 12, 13, 14 and Tables 3, 4, 5.
Gas temperature used in the 0D model for the MW plasma, as a function of gas flow rate, based on the 2D simulations of Fig. 13
Rights and permissions
About this article
Cite this article
Zhang, QZ., Wang, W., Thille, C. et al. H2S Decomposition into H2 and S2 by Plasma Technology: Comparison of Gliding Arc and Microwave Plasma. Plasma Chem Plasma Process 40, 1163–1187 (2020). https://doi.org/10.1007/s11090-020-10100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10100-3