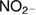Abstract
In recent years, the interest in treating cancer cells with plasma treated media (PTM) and plasma treated water (PTW) has increased tremendously. However, the actions of PTM and PTW are still not entirely understood. For instance, it is not clear whether the action of PTM is due to a modification in proteins/amino acids after plasma treatment of the media, or due to reactive oxygen and nitrogen species (RONS) generated from the plasma, or a combination of both effects. To differentiate between the actions of RONS and modified proteins/amino acids on the treatment of cancer cells, we compared the effects of PTM and PTW on two different pancreatic ductal adenocarcinomas (MiaPaca-2, BxPc3) and pancreatic stellate cells (PSCs) (hPSC128-SV). PSCs closely interact with cancer cells to create a tumor-promoting environment that stimulates local tumor progression and metastasis. We treated culture media and deionized water with a cold atmospheric plasma (CAP) jet, and subsequently applied this PTM/PTW at various ratios to the pancreatic cancer and PSC cell lines. We evaluated cell death, intracellular ROS concentrations and the mRNA expression profiles of four oxidative stress-related genes, i.e. Mitogen-activated protein kinase 7 (MAPK7), B-cell lymphoma 2 (BCL2), Checkpoint kinase 1 (CHEK1) and DNA damage-inducible transcript 3, also known as C/EBP homologous protein (CHOP). Our findings demonstrate that PTM and PTW have a similar efficacy to kill pancreatic cancer cells, while PTW is slightly more effective in killing PSCs, as compared to PTM. Furthermore, we observed an enhancement of the intracellular ROS concentrations in both pancreatic cancer cells and PSCs. Thus, it is likely that under our experimental conditions, the anti-cancer activity of PTM can be attributed more to the RONS present in the treated liquid, than to the modification of proteins/amino acids in the media. Furthermore, the fact that the chemo-resistant PSCs were killed by PTM/PTW may offer possibilities for new anti-cancer therapies for pancreatic cancer cells, including PSCs.
Export citation and abstract BibTeX RIS
Introduction
Cold atmospheric plasma (CAP) shows significant potential for various biomedical applications, such as for sterilization of infected tissue [1, 2], inactivation of microorganisms [3], wound healing [2], skin regeneration [4], blood coagulation [5], tooth bleaching [6], and cancer therapy [7]. CAP produces various reactive oxygen and nitrogen species (RONS), which contribute to the selective killing of a variety of cancer cells [8, 9]. However, direct plasma treatment is limited to tumors at the upper surface of the human body [7, 10]. Recent reports suggest that indirect plasma treatment, by means of plasma treated liquids (PTLs) (phosphate buffered solution, deionized water, culture media, etc), has similar anti-cancer activity [7, 10–12]. Therefore, to increase the use of plasma in cancer treatment, especially for internal organ tumor treatment, plasma treated solutions may be an interesting alternative cancer therapy. Indeed, plasma treated solutions can be used to target tumors deep inside the body, where direct plasma treatment is not possible due to gas delivery, high voltage and generation of discharge inside the human organs [13].
One reason why CAP is promising for cancer therapy is because it generates RONS, which execute essential cellular functions in living organisms [12]. Many types of cancer cells exhibit elevated levels of RONS, and thus cancer cells can be killed by enhancing the intracellular RONS concentrations [10, 14]. CAP generates RONS in the gas phase, but when plasma irradiates a liquid surface, these RONS are transported into the liquid. When the liquid is a cell culture medium, the RONS undergo further reactions with the dissolved organic molecules (i.e. amino acids, proteins and sugars) in the liquid [15, 16]. Furthermore, the reaction between the plasma-generated RONS and water molecules results in the production of relatively long-lived reactive species, such as hydrogen peroxide (H2O2), nitrate ( ), and nitrite (
), and nitrite ( ) ions. Eventually these RONS are thought to function as moderators or effectors in cell signaling cascades [17, 18].
) ions. Eventually these RONS are thought to function as moderators or effectors in cell signaling cascades [17, 18].
Pancreatic cancer is the fourth leading cause of cancer-related deaths worldwide [19–21], and it is projected to be the second leading cause of cancer-related deaths by 2030 [22]. Despite extensive research efforts over the past decades, surgical resection with macroscopic tumor clearance, chemotherapy or radiotherapy are currently the only available treatments for pancreatic cancer [19–21]. However, most of the patients are diagnosed at an advanced stage, when metastasis has already started, and during that stage only 10%–20% of patients that can undergo surgery are cured [20] and the remaining 80%–90% of the patients are limited to chemo- and radiotherapy [21]. The chemotherapeutic drugs ergotamine and gemcitabine were approved for the treatment of advanced pancreatic cancer stages [23, 24], however, their survival benefit is small and the cost is high [24]. Even with combined treatment of radio-chemotherapy, the survival rate is very low [19, 20]. On the other hand, the use of only radiotherapy in locoregionally advanced pancreatic cancer remains controversial because of side effects such as pain, biliary obstruction, bleeding, and bowel obstruction [25]. The current limitations of these therapies increase the likelihood of mortality in pancreatic cancer patients, affirming the need for new efficacious therapeutic options such as plasma treatment.
Some reports have shown that direct and indirect application of CAP induces cell death in pancreatic cancer cells, i.e. Colo-357, PaTu8988T, and 6606PDA cells [26–29], but there is currently no evidence of CAP being able to kill PSCs, such as hPSC128-SV. It should be noted that hPSC128-SV produces a stromal reaction, which provides a unique tumor microenvironment in pancreatic cancer that is responsible for chemo-resistance in pancreatic cancer [30–35].Therefore, in our study, we treated culture medium (Dulbecco Modified Eagle Medium; DMEM) and deionized (DI) water with the kINPen® IND plasma jet, and applied this plasma treated medium (PTM) and plasma treated water (PTW) to pancreatic cancer cells (Miapaca-2 and Bxpc3), and also to human pancreatic stellate cells (PSCs; hPSC128-SV).
To obtain a better insight into the mechanisms of cell death upon treatment with PTM/PTW, we also investigated the changes in intracellular ROS concentrations and mRNA expression profiles on four oxidative stress-related genes, i.e. Mitogen-activated protein kinase 7 (MAPK7), B-cell lymphoma 2 (BCL2), Checkpoint kinase 1 (CHEK1) and DNA damage-inducible transcript 3, also known as C/EBP homologous protein (CHOP), after treatment with PTM and PTW.
The overall aim of our study was to compare the anti-cancer activities of both PTM and PTW against pancreatic cancer cells and also against chemo-resistant PSCs. Furthermore, this comparison enables the determination of whether the action of PTM is due to modifications in proteins/amino acids after plasma treatment of the medium, or is only due to the presence of RONS in the liquid, as in PTW, or a combination of both.
Methodology
Plasma device
The kINPen® IND plasma jet (INP Greifswald/neoplas tools GmbH, Greifswald, Germany) was used. Detailed information about the design and operation of the kINPen® IND plasma device was reported previously [2, 11]. Briefly, it houses two electrodes: a pin electrode (1 mm diameter) in the center that is separated by a dielectric capillary (1.6 mm inner diameter) from a grounded ring electrode. The plasma is generated by high frequency sinusoidal voltage of around 2–6 kVpp to the central electrode, with a frequency between 1.0 and 1.1 MHz and a maximum power of 3.5 W. To control the temperature, the device operates as a switch off and on mode with a frequency of 2.5 kHz. The plasma is created inside the capillary and creates a plasma effluent with a length of 9–12 mm from the 1 mm diameter with the gas flow towards the open side of the device [2, 11]. For the plasma treatments, we used argon gas (purity 99.9%) with a flow rate of 3 lpm, and we kept a 10 mm distance between the nozzle of the plasma jet device and the liquid surface [11]. Based on the literature [36, 37], the electron density of the kINPen® IND plasma jet was in the order of 1012 cm3, the power density was 1.5 W cm−3 and the gas temperature was in the range of 45 °C–50 °C. The optical emission spectra revealed excited argon peaks between 700 and 900 nm, nitrogen emission lines between 330 and 420 nm, as well as OH emission at 309 nm, using optical emission spectroscopy as reported previously [38].
Sample preparation for plasma treatment
To make the PTM and PTW, DI water or DMEM was treated with plasma for 3, 5 and 10 min in a 48-well plate. We analyzed the pH and H2O2/ concentrations in the plasma treated solutions, using the method described below. For the cell viability, apoptosis and gene expression, we used 10 min exposed PTM/PTW. After exposure, 300 µl of PTM/PTW remained of the initial 800 µl of culture media or water. Subsequently, we used different percentages, i.e. 50, 25, 12.5, 6.2, 3.1 and 1.5%, of the PTM or PTW, which we added to a 48-well plate, containing cells with untreated culture media. After addition of the respective percentages of PTM/PTW, the total volume was 500 µl per well. Next, we incubated the treated/non-treated cells for 24, 48 and 72 h. In this study, the abbreviations PTM/PTW-50, PTM/PTW-25, PTM/PTW-12.5, PTM/PTW-6.2, PTM/PTW-3.1 and PTM/PTW-1.5 will be used for the 50, 25, 12.5, 6.2, 3.1 and 1.5% of PTM/PTW (formed after 10 min plasma treatment), respectively.
concentrations in the plasma treated solutions, using the method described below. For the cell viability, apoptosis and gene expression, we used 10 min exposed PTM/PTW. After exposure, 300 µl of PTM/PTW remained of the initial 800 µl of culture media or water. Subsequently, we used different percentages, i.e. 50, 25, 12.5, 6.2, 3.1 and 1.5%, of the PTM or PTW, which we added to a 48-well plate, containing cells with untreated culture media. After addition of the respective percentages of PTM/PTW, the total volume was 500 µl per well. Next, we incubated the treated/non-treated cells for 24, 48 and 72 h. In this study, the abbreviations PTM/PTW-50, PTM/PTW-25, PTM/PTW-12.5, PTM/PTW-6.2, PTM/PTW-3.1 and PTM/PTW-1.5 will be used for the 50, 25, 12.5, 6.2, 3.1 and 1.5% of PTM/PTW (formed after 10 min plasma treatment), respectively.
Measurement of pH and H2O2/ concentrations in the plasma treated solutions
concentrations in the plasma treated solutions
To determine the pH of the solution after plasma exposure, DMEM and DI water were exposed to CAP for up to 10 min. Just after exposure, the pH of the PTM/PTW was measured using a pH meter (pHenomenalR pH 1100 H pH-meter (VWR)). The hydrogen peroxide (H2O2) concentrations in the medium and water were analyzed after CAP exposure at different time intervals using the titanyl ion as described previously [11], while the  concentration was measured using the Griess Reagent Nitrite Measurement kit (Cell Signaling Technology®, 13547), as described previously [11].
concentration was measured using the Griess Reagent Nitrite Measurement kit (Cell Signaling Technology®, 13547), as described previously [11].
Analysis of cell death
The cytotoxicity of PTM/PTW on two types of pancreatic ductal adenocarcinomas (MiaPaca-2, BxPc3), and hPSC128-SV pancreatic stellate cells (PSCs) was evaluated. MiaPaca-2 and hPSC128-SV cells were maintained in DMEM supplemented with 10% FBS, 1% non-essential amino acids, 1% glutamine, 1% penicillin (100 IU ml−1) and streptomycin (100 mg ml−1) (all from Gibco™ DMEM, Life Technologies), whereas Bxpc3 cells were cultured in RPMI supplemented with 10% FBS, 1% non-essential amino acids, 1% glutamine, 1% penicillin (100 IU ml−1) and streptomycin (100 mg ml−1) (all from Gibco™ DMEM, Life Technologies). All cultures were maintained at 37 °C, 95% relative humidity and 5% CO2. The cells were grown in 75 cm2 tissue culture flasks until they reached confluence and were then sub-cultured for the experiments. 2–4 × 105 cells ml−1 were seeded into 48-well plates to grow in complete media. PTM/PTW-50, PTM/PTW-25, PTM/PTW-12.5, PTM/PTW-6.2, PTM/PTW-3.1 and PTM/PTW-1.5 were added to each well, as described above, to obtain a final volume of 500 µl. We used equation (1), to calculate the concentration of H2O2 transfer to each well during the PTM/PTW treatment:
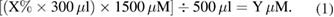
To check the cell viability, each plate was carefully rinsed with PBS. The viability was assessed using 50 µl MTT (3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide) solutions (5 mg ml−1 in PBS), which was added to each well in the plate, and the plates were incubated for 3 h. Following incubation, the medium was removed and the purple formazan precipitates in each well were released in the presence of 500 µl DMSO (dimethyl sulphoxide). The absorbance was measured using a microplate reader (BIO-RAD iMark Microplate reader) at 540 nm and the cell viability was assessed as the absorbance ratio between the PTM/PTW treated and control cells, which was directly proportional to the number of metabolically active cells.
Furthermore, the MiaPaca-2, BxPc3, and hPSC128-SV cells were exposed to PTM/PTW-12.5, to detect cell death using the Annexin V-FITC apoptosis detection kit (FITC Annexin V Apoptosis Detection Kit, BD Biosciences). The treated/untreated cells were washed with 1 ml of cold 1X binding buffer after a 3 h incubation, and were subsequently trypsinized. Annexin V-FITC (0.5 mg ml−1) was added to each sample. After incubation for 15 min at room temperature, the cells were again washed with PBS and stained with 0.3 mg ml−1 of PI (Propidium Iodide) and analyzed using flow cytometry (Attune NxT Flow Cytometer).
Intracellular ROS estimation
To investigate the total ROS content inside the cells, the CellROX™ Green molecular probe was used. 3000 cells/well (96 well-plate) were exposed to PTM/PTW-12.5 with 50 µM CellROX™ Green. We used real time measurements for 10 h using the IncuCyte ZOOM® system control (Essen Bioscience) microplate reader.
RNA extractions for quantitative real time PCR
To perform a quantitative evaluation of the expression of oxidative related genes, the total RNA content was isolated from the untreated and treated cells after a 3 h exposure with PTM/PTW-12.5, using the Purelink™ RNA Mini kit (Ambion®) according to the manufacturer's protocol. The RNA concentrations were measured using an Epoch Microplate Spectrophotometer (BioTek®). Before Real-Time PCR, the RNA samples were treated with RQ1 Rnase-Free Dnase (1 unit µg−1 RNA) (Promega©). cDNA was synthesized from 2 µg RNA, using the SuperScript® II Reverse Transcriptase (Invitrogen™). PCR reactions were performed using the Step One Plus™ Real-Time PCR (Applied Biosystems™, Foster City, CA, USA). cDNA samples were diluted to a concentration of 5 ng µl−1. Primers were diluted to a stock of 5 µM. Real-Time PCR was conducted using 10 ng cDNA, 1× SYBR Green I (Invitrogen™), 150 nM forward and reverse primer:
- GAPDH (F-GGAAGCATAAGAACATCGGAACTG, R-GGAGTGGGTGTCGCTGTTG),
- HPRT1 (F-TGACACTGGCAAAACAATGCA, R-GGTCCTTTTCACCAGCAAGCT),
- MAPK7 (F-CAGAGTCACCTGATGTCAACCTT, R-GGGTCCTCCACCTGTGACT),
- BCL2 (F-GGTGAACTGGGGGAGGATTGT, R-CTTCAGAGACAGCCAGGAGAA),
- CHEK1 (F-TTGTGAGCAGCCAGAAGATTT, R-GCAGCACTATATTCACCAGGATT),
- CHOP (F- TGCTTCTCTGGCTTGGCTGAC, R-CCGTTTCCTGGTTCTCCCTTGG).
All reactions were performed on a standard run for 2 h using the following program: Denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Finally, melting curves of the amplicons were acquired by heating the samples for 15 s–95 °C, and for 1 min to 60 °C. The relative expression was determined using the comparative ΔΔt-method and qBase + software (Biogazelle). Reference genes GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) and HPRT1 (Hypoxanthine phosphoribosyl-transferase I) were selected from a list of 10 reference targets using the geNorm algorithm.
Results and discussion
Determination of pH and H2O2/ concentrations in PTM/PTW
concentrations in PTM/PTW
To determine the change in properties of PTM and PTW after exposure to CAP, the pH and the concentrations of H2O2 and  were measured at different exposure times and dilutions. As demonstrated in figure 1(b), after 10 min of treatment, there was a drastic decrease in the pH of PTW, while only a small increase in pH was observed for PTM. After the addition of PTM/PTW-50, 25, 12.5 and 6.2 to the cell culture media, a slight reduction in the pH was observed after addition of PTW-50 to the media, but in all other cases there was no change in pH (figure 1(c)). Therefore, to avoid the effect of pH on cell toxicity, we did not use PTW-50 in our further experiments.
were measured at different exposure times and dilutions. As demonstrated in figure 1(b), after 10 min of treatment, there was a drastic decrease in the pH of PTW, while only a small increase in pH was observed for PTM. After the addition of PTM/PTW-50, 25, 12.5 and 6.2 to the cell culture media, a slight reduction in the pH was observed after addition of PTW-50 to the media, but in all other cases there was no change in pH (figure 1(c)). Therefore, to avoid the effect of pH on cell toxicity, we did not use PTW-50 in our further experiments.
Figure 1. (a) Schematic representation of PTM/PTW production using a CAP jet source; (b) measurement of pH after 10 min direct exposure of CAP in DI water and DMEM; (c) measurement of pH after adding different percentages of PTW and PTM to cell culture media; measurement of (d)  and (e) H2O2 concentrations after 3, 5 and 10 min plasma exposure in DI water and DMEM. All measurements were performed in triplicate and the values are plotted as the mean ± standard deviation.
and (e) H2O2 concentrations after 3, 5 and 10 min plasma exposure in DI water and DMEM. All measurements were performed in triplicate and the values are plotted as the mean ± standard deviation.
Download figure:
Standard image High-resolution imageAs shown in figures 1(d) and (e), the  and H2O2 concentrations generally increased upon longer CAP treatment times in both PTM and PTW, although the increase in
and H2O2 concentrations generally increased upon longer CAP treatment times in both PTM and PTW, although the increase in  concentration in PTW was not very pronounced. At 3 and 5 min of treatment time, both the
concentration in PTW was not very pronounced. At 3 and 5 min of treatment time, both the  and H2O2 concentrations were somewhat higher in PTW than in PTM. This might be because upon increasing plasma treatment more and more
and H2O2 concentrations were somewhat higher in PTW than in PTM. This might be because upon increasing plasma treatment more and more  is converting to
is converting to  , or to other reaction products due to a reaction of short-lived radicals with
, or to other reaction products due to a reaction of short-lived radicals with  , and therefore the concentration of
, and therefore the concentration of  decreases. On the other hand, in PTM less
decreases. On the other hand, in PTM less  is converting to
is converting to  or other products, because the short-lived radicals that would react with
or other products, because the short-lived radicals that would react with  may react faster with the amino acids/proteins in the PTM, resulting in more
may react faster with the amino acids/proteins in the PTM, resulting in more  in PTM. However, the concentration of H2O2 is clearly much higher than the
in PTM. However, the concentration of H2O2 is clearly much higher than the  concentration in all treatment conditions, and similar trends were also reported in a previous study [11].
concentration in all treatment conditions, and similar trends were also reported in a previous study [11].
Recently, Van Boxem et al [11] demonstrated that for a small gap (i.e. the distance between the plasma device outlet and PBS solution) and high gas flow rates of feeding gas, the concentration of H2O2 is high as compared to the  concentration; because at this condition, a large fraction of gaseous OH radicals reaches the liquid, resulting in a higher H2O2 concentration in the liquid. However, at a large gap and low flow rates of feeding gas, the concentration of H2O2 is comparable to
concentration; because at this condition, a large fraction of gaseous OH radicals reaches the liquid, resulting in a higher H2O2 concentration in the liquid. However, at a large gap and low flow rates of feeding gas, the concentration of H2O2 is comparable to  , because many of the gaseous OH radicals will readily recombine in the gas phase and a lower amount of H2O2 reaches the liquid surface. On the other hand, most N-species (such as NO or NO2) reach the liquid phase, because their gas density is much higher than that of the O-species, as 80% of ambient air consists of N2. In this study we also used high gas flow rates and a small gap, yielding a high H2O2 concentration compared with the
, because many of the gaseous OH radicals will readily recombine in the gas phase and a lower amount of H2O2 reaches the liquid surface. On the other hand, most N-species (such as NO or NO2) reach the liquid phase, because their gas density is much higher than that of the O-species, as 80% of ambient air consists of N2. In this study we also used high gas flow rates and a small gap, yielding a high H2O2 concentration compared with the  concentration, which is in good agreement with [11].
concentration, which is in good agreement with [11].
Effects of PTM and PTW on cell viability
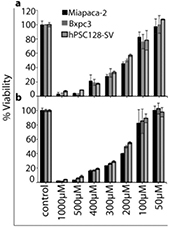
The MTT assay was used to determine the cell viability of PTM and PTW treated pancreatic cancer cells (Miapaca-2, Bxpc3) and human PSCs (hPSC128-SV). For untreated controls, the same percentages of non-treated media (NTM) and DI water (NTW) were added. As shown in figure 2, all three cell lines demonstrated a slight decrease in viability after treatment with PTM/PTW-3.1, but the reduction in viability was quite pronounced for PTM/PTW-25, PTM/PTW-12.5 and PTM/PTW-6.2, after incubation for 24, 48, and 72 h, certainly in comparison with NTM and NTW. The addition of NTW-25 decreased the cell viability to some extent for all three studied cell lines, due to dilution of the culture media (see figure 2).
Figure 2. Change in cell viability after 24, 48 and 72 h incubation with different percentages of PTW and PTM added to (a) Miapaca-2 cells, (b) Bxpc3 cells and (c) hPSC128-SV cells. In addition, the results are shown for the control cells (without treatment) and for cells to which non-treated media and water (NTM/NTW) were added. All measurements were performed in triplicate and the values are plotted as the mean ± standard deviation.
Download figure:
Standard image High-resolution imageWhen comparing the effects on the three different cell lines, we observed a cytotoxicity of ≈85%, after treatment of the Miapaca-2 and Bxpc3 cells with PTM/PTW-25 while at the same concentration of PTM/PTW treatment of the hPSC128-SV cells only ≈60% cytotoxicity was observed after 24 h of incubation, as shown in figure 2(c). This demonstrates that the hPSC128-SV cells, which are responsible for the strong desmoplastic reaction (i.e. PSC cells are associated with pancreatic tumors and they closely interact with cancer cells to create a tumor-promoting environment that stimulates local tumor progression and metastasis), are more resistant. However, when the incubation time increases to 48 and 72 h, about 70% and 80% of the hPSC128-SV cells are dead, respectively, as well as more than 90% of the Miapaca-2 and Bxpc3 cells.
When comparing the effects of PTM and PTW, it is clear that they generally have a similar killing capacity for the Miapaca-2 and Bxpc3 cancer cells, with sometimes PTW being more effective and sometimes vice versa. However, PTW seems to be slightly more effective in deactivating the hPSC128-SV cells than PTM. Thus, our results show that the cell killing capacity of PTM is not due to a modification in proteins/amino acids after plasma treatment of the medium, but rather due to the presence of RONS in the liquid, as in PTW.
To determine the role of H2O2 on the viability of Miapaca2, Bxpc3 and hPSC128-SV cells, we treated these cell lines with different concentrations of H2O2 and incubated them for 24 and 48 h, as shown in figure 3. The viability of the cancer cells after 100 µM of H2O2 treatment was 85, 80 and 81% for the Miapaca2, Bxpc3 and hPSC128-SV cells, respectively, after 24 h of incubation (figure 3). We added different percentages (25, 12.5, 6.2, 3.1 and 1.5%) of PTM/PTW to the culture media (untreated) with cells, to obtain a final volume of 500 µl in each well, as described above, thus yielding H2O2 concentrations of 225 µM, 112.5 µM, 55.8 µM, 22.9 µM and 13.5 µM, respectively. The calculation of the final H2O2 concentrations present in the total cell media (500 µl) was described in the Methodology section. When comparing the viability of the above-mentioned cancer cells after treatment with PTM/PTW-12.5 (yielding a H2O2 concentration of 112.5 µM), we observed approximately 25% viability in the Miapaca-2 and Bxpc3 cells, and approximately 40% cell viability in the hPSC128-SV cells (see figure 2), which is much lower than the viability of ca. 80%–85% after direct treatment with 100 µM of H2O2 (see figure 3). Hence, we can conclude that the PTM/PTW action was stronger and was not only due to the H2O2 effect.
Figure 3. Change in cell viability of Miapaca-2, Bxpc3 and hPSC128-SV cells after (a) 24 and (b) 48 h incubation with different µM concentration of H2O2. All measurements were performed in triplicate and the values are plotted as the mean ± standard deviation.
Download figure:
Standard image High-resolution imageAdditionally, we tried to minimize the effects of osmotic-pressure change. Therefore, our experimental data (figure 2) was based on the comparative study with different percentages of non-plasma treated media and water (NTM/NTW). We found only 10%–15% cell death for the 25% non-PTW, while in the other treatment conditions, i.e. 12.5, 6.2, 3.1 and 1.5% non-PTW, we did not find any cell death. Hence, there was only a slight effect of the osmotic-pressure with the addition of 25% non-PTW. To avoid the effect of osmotic pressure caused by PTW, we used 12.5% of PTW in our experiments, as discussed below.
Furthermore, we also analysed the degree of apoptosis/necrosis after treatment of all three cell types. For this purpose, we incubated the cells with PTM/PTW-12.5 for 3 h, after which the cells were harvested, and stained with annexin V-FITC/PI. The majority of dead cells stained both annexin V-FITC and PI, indicating that the PTM/PTW-treated non-viable cells were mostly late apoptotic or necrotic. In late apoptosis, loss of membrane integrity will allow PI to intercalate to DNA (Annexin V positive, PI positive) and in the necrotic process, the membrane integrity is lost and PI will intercalate to DNA (Annexin V negative, PI positive). However, Annexin V will enter this permeated cell membrane in necrosis in order to bind to PS on the inner cell membrane (Annexin V positive, PI positive). Therefore, in the current experimental setup, Annexin V and PI positive staining represents late apoptotic combined with necrotic cell death [12, 39].. As shown in figure 4, for Miapaca-2, PTM-12.5 induced ≈80% late apoptosis/necrosis (Annexin V positive, PI positive), while PTW-12.5 induced ≈70% late apoptosis/necrosis (figure 4(d)). For Bxpc3, PTM-12.5 induced ≈70% late apoptosis/necrosis, while PTW-12.5 induced ≈65% late apoptosis/necrosis, (figure 4(e)). Finally, for the hPSC128-SV cells, PTM-12.5 induced only ≈40% late apoptosis/necrosis, and PTW-12.5 induced ≈60% late apoptosis/necrosis, (figure 4(f)). Again, these results indicate that PTW action on hPSC128-SV is stronger than PTM, as shown in figure 2.
Figure 4. Flow cytometry analysis of the (a) Miapaca-2, (b) Bxpc3, and (c) Hpsc128-SV cells in the presence of PTM/PTW-12.5 and H2O2, as well as control cells (without treatment); % cell death of (d) Miapaca-2; (e) Bxpc3 and (f) hPSC128-SV cells, upon treatment with PTM/PTW-12.5 and H2O2.
Download figure:
Standard image High-resolution imageTaken together, these results demonstrate that CAP treated media and DI water can induce apoptosis/necrosis in pancreatic cancer cells, and also in chemo-resistant stellate cells. The H2O2 solutions (100 µM) also induce ≈25% late apoptosis/necrosis in the Miapaca-2 and Bxpc3 cell lines, while in the hPSC128-SV cells ≈30% late apoptosis/necrosis was observed. As this is lower than the effects of PTW and PTM, this also demonstrates that the reduction in cancer cell viability was not only due to the presence of H2O2, but that it was a combined effect of various RONS that were produced in the PTM/PTW, which eventually influence intracellular redox signaling through enhancement of intracellular ROS.
Intracellular ROS and oxidative stress-related gene analysis after PTM/PTW treatment of the cancer cells
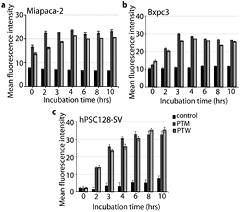
The intracellular ROS levels were estimated using fluorescent probes. The intensity of the CellROX™ Green probe, used to detect the intracellular ROS content, dramatically increased upon addition of PTM/PTW-12.5, and a higher fluorescence intensity was detected for all three treated cell lines in comparison with the control, as shown in figure 5. The enhancement of the intracellular ROS content might be due to the CAP generated extracellular RONS. In turn, the increase in intracellular ROS content would result in higher oxidative stress inside the cell, and this could modulate several protein signaling pathways, such as the apoptotic pathway [40].
Figure 5. Real-time based intracellular ROS content estimation for up to 10 hr incubation for (a) Miapaca-2, (b) Bxpc3 and (c) hPSC128-SV cells in the presence of PTM/PTW-12.5, as well as for control cells (without treatment). All measurements were performed in triplicate and the values are plotted as the mean ± standard deviation.
Download figure:
Standard image High-resolution imageTo better understand the latter effects, we evaluated the mRNA expression profiles of four oxidative stress and apoptotic-related genes, i.e. MAPK7, BCL2, CHEK1 and CHOP, as shown in figure 6. MAPK7 has been suggested to be a potential tumor biomarker for many cancers, as an abnormal upregulation of MAPK7 is involved in cell proliferation and metastasis in several types of cancer [41]. Furthermore, the BCL2 family of proteins are one of the key regulators of cell death by apoptosis [42, 43] and a high expression of BCL2 mediates the resistance of various cancers to a wide range of chemotherapeutic drugs and γ-irradiation [42]. Therefore, downregulation of MAPK7 and BCL2 can restore the apoptotic process in tumor cells. CHEK1 plays an essential role in the cell cycle checkpoint response following DNA damage, and targeting these regulators by inhibition or depletion would sensitize cancer cells to chemotherapy [44, 45]. Hence, downregulation of CHEK1 induces double-stranded DNA breaks and chromosomal fragmentation in cancer cells. Finally, expression of CHOP is upregulated under endoplasmic reticulum (ER) stress and this would also lead to apoptosis [46]. In our study, the PTM/PTW treatment (see figure 5) downregulated the expression of the tumor biomarkers, MPK7, BCL2 and CHEK1 in all three pancreatic cells (see figure 6), which could explain the increase in cell death. This is in agreement with previous reports [39–44]. However, the expression of CHOP did not change significantly upon PTM/PTW treatment.
Figure 6. Relative value of mRNA expression (compared to control cells) of four apoptosis-related genes in Miapaca-2, Bxpc3 and hPSC128-SV cells, after exposure with PTM/PTW-12.5 and H2O2. The relative mRNA expression of (a) MAPK7, (b) BCL2, (c) CHK1, and (d) CHOP was measured by real-time PCR after 3 h of incubation. All measurements were performed in triplicate and the values are plotted as the mean ± standard deviation.
Download figure:
Standard image High-resolution imageBased on previous reports, the major effects from plasma exposure were found to be the activation of oxidative mediated pathways such as MAPK and p53 signaling pathways, resulting in changes in apoptosis related gene expression [47]. Additionally, antioxidant machinery such as nrf2-involved in regulation of heme oxygenase 1, and NADPH-quinone oxidoreductase [48]. Some previous studies have shown that oxidative stress elicits cancer cell death through the inactivation of MAPK7, CHEK1 and BCL2 expression [41–44]. It could be likely that PTM/PTW plays a major role in initiating cell death in pancreatic cancer cells (Miapaca-2 and Bxpc3), as well as in the chemo-resistant human PSCs (hPSC128-SV) through down regulation of MAPK7, BCL2 and CHEK1 gene expression, and also oxidative stress mediated pathways.
The above mentioned experimental results demonstrate that the treatment of pancreatic ductal adenocarcinomas and PSCs by PTM/PTW deactivates these cells, but the deactivation strongly depends on the concentrations of PTM/PTW used. PTM/PTW mainly consists of long-lived species, such as H2O2,  and
and  , and among them H2O2 and
, and among them H2O2 and  are of main interest for cancer cell death [49–51]. Some studies have shown that the action of PTL against cancer cells is most likely as H2O2 treatment [52], whereas in other studies it was reported that the presence of both ROS and RNS in PTL are responsible for cancer cell death [49, 53]. It was also reported that the long lifetime species such as H2O2 and
are of main interest for cancer cell death [49–51]. Some studies have shown that the action of PTL against cancer cells is most likely as H2O2 treatment [52], whereas in other studies it was reported that the presence of both ROS and RNS in PTL are responsible for cancer cell death [49, 53]. It was also reported that the long lifetime species such as H2O2 and  fully account for the toxicity of plasma [49–51]: H2O2 seems to play a key role in cell death, but the production of
fully account for the toxicity of plasma [49–51]: H2O2 seems to play a key role in cell death, but the production of  along with H2O2 increases the toxicity [49–51, 53]. The presence of H2O2 and
along with H2O2 increases the toxicity [49–51, 53]. The presence of H2O2 and  in PTM/PTW leads to the generation of ONOO− [49, 54], which has a high potential to kill cancer cells [55]. On the other hand, Bauer recently suggested that singlet oxygen is the main component of cell death in PTM treatment [51]. It is generated by a complex reaction between ONOO− and H2O2 [51]. The above studies show that the cytotoxicity of PTM/PTW is not only due to the H2O2 effect, but that it is a combined effect of H2O2 and
in PTM/PTW leads to the generation of ONOO− [49, 54], which has a high potential to kill cancer cells [55]. On the other hand, Bauer recently suggested that singlet oxygen is the main component of cell death in PTM treatment [51]. It is generated by a complex reaction between ONOO− and H2O2 [51]. The above studies show that the cytotoxicity of PTM/PTW is not only due to the H2O2 effect, but that it is a combined effect of H2O2 and  , which results in many direct and indirect reactions taking place during PTM/PTW treatment. Our study also demonstrates that H2O2 is not the only factor for cancer cell death, but that it could be a combination of H2O2 and
, which results in many direct and indirect reactions taking place during PTM/PTW treatment. Our study also demonstrates that H2O2 is not the only factor for cancer cell death, but that it could be a combination of H2O2 and  effects.
effects.
Conclusion
In our study, RONS generated by CAP treatment of both culture medium and DI water showed a remarkable effect on the cell viability of pancreatic cancer cells, including stellate cells (hPSC128-SV). The fact that PTM did not yield more cancer cell death than PTW, and was even slightly less effective for stellate cells, suggests that part of the RONS present in PTM could be used for the reaction with amino acids/proteins, which are present in the cell media [16] and may not be available anymore for killing the cancer cells. Furthermore, it also shows that the modified amino acids/proteins upon reaction with RONS did not seem to have a strong anti-cancer action. Additionally, our data suggest that the killing effect was more than only due to H2O2, as the PTM/PTW were more effective in killing the three cell lines than H2O2 solutions with similar concentrations. Furthermore, the PCR results showed that PTM/PTW likely induces oxidative stress-related cell death in the three cell lines, i.e. Miapaca2, Bxpc3, and hPSC128-SV, by causing downregulation of MPK7, BCL2, and CHEK1 expression. Finally, our findings demonstrate that PTM/PTW could be a potential new anti-cancer drug for pancreatic cancer, as it also appears to be effective in chemo-resistant PSCs (hPSC128-SV).
Acknowledgments
We gratefully acknowledge financial support from the Research Foundation—Flanders (FWO) (grant number 12J5617N) and from the European Marie Skłodowska–Curie Individual Fellowship 'Anticancer-PAM' within Horizon2020 (grant number 743546). We also thank Atsushi Masamune (Division of Gastroenterology, Tohoku University Graduate School of Medicine, Sendai, Miyagi Prefecture, Japan) for providing us with human PSCs (hPSC128-SV) for this study.