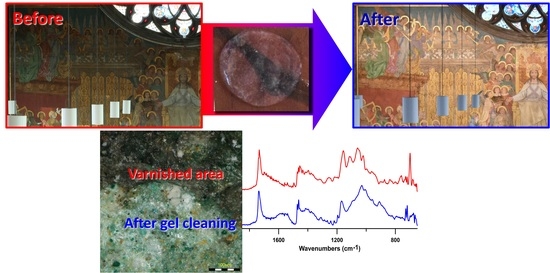
Removal of a Past Varnish Treatment from a 19th-Century Belgian Wall Painting by Means of a Solvent-Loaded Double Network Hydrogel
Abstract
:1. Introduction
Historical Context
2. Materials and Methods
2.1. Materials
2.2. Instrumentations
2.2.1. Optical Microscopy (OM)
2.2.2. SEM-EDX
2.2.3. XRD
2.2.4. FTIR Spectroscopy
2.3. Sample Preparation
3. Results and Discussions
3.1. Materials and Structure of the Wall Painting
3.1.1. Painting Stratigraphy and Technique
3.1.2. Conservation State
3.2. Gel Cleaning Test Strategy
3.3. Varnish Identification
3.4. Laboratory Tests
3.4.1. Free Solvents Solubility/Swelling Tests on the Collected Varnish Fragments
3.4.2. Hydrogel Swelling Tests on the Collected Varnish Fragments
3.5. Gel Cleaning Tests on the Wall Painting
3.5.1. First Stage
3.5.2. Second Stage
3.5.3. Third Stage
3.6. Implementation of Gel Cleaning Treatment of the Wall Painting and Verification of Varnish Removal
3.7. Final Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, K. Wall Paintings: Aspects of Deterioration and Restoration. In Conservation Science: Heritage Materials; May, E., Jones, M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2006; pp. 241–265. [Google Scholar]
- Horie, V. Materials for Conservation: Organic Consolidants, Adhesives and Coatings, 2nd ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Mora, P.; Mora, L.; Philippot, P. Conservation of Wall Paintings; Butterworths: London, UK, 1984. [Google Scholar]
- Mazzeo, R.; Sciutto, G.; Bonacini, I.; Prati, S. Scientific Examinations of the Armenian Church Wall Paintings in Famagusta. In The Armenian Church of Famagusta and the Complexity of Cypriot Heritage: Prayers Long Silent; Walsh, M.J.K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 269–283. [Google Scholar]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Stulik, D.; Dorge, V.; Miller, D.; Khanjian, H.; Institute, G.C.; Carlson, J.; Khandekar, N.; Wolbers, R.; Petersen, W.C. Solvent Gels for the Cleaning of Works of Art: The Residue Question; Getty Publications: Los Angeles, CA, USA, 2004. [Google Scholar]
- Michalski, S. A physical model of the cleaning of oil paint. Stud. Conserv. 1990, 35, 85–92. [Google Scholar] [CrossRef]
- Baglioni, M.; Jáidar Benavides, Y.; Desprat-Drapela, A.; Giorgi, R. Amphiphile-based nanofludis for the removal of styrene/acrylate coatings: Cleaning of stucco decoration in the Uaxactun archeological site (Guatemala). J. Cult. Herit. 2015, 16, 862–868. [Google Scholar] [CrossRef]
- Baglioni, M.; Jàidar Benavides, Y.; Berti, D.; Giorgi, R.; Keiderling, U.; Baglioni, P. An amine-oxide surfactant-based microemulsion for the cleaning of works of art. J. Colloid Interface Sci. 2015, 440, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Guizzo, S.; Tortolini, C.; Pepi, F.; Leonelli, F.; Mazzei, F.; Di Turo, F.; Favero, G. Application of microemulsions for the removal of synthetic resins from paintings on canvas. Nat. Prod. Res. 2019, 33, 1015–1025. [Google Scholar] [CrossRef]
- Andreotti, A.; Brown, W.P.; Camaiti, M.; Colombini, M.P.; DeCruz, A. Diagnosis of materials and effectiveness of Er:YAG laser cleaning as complementary treatment in a panel painting attributed to Lluís Borrassà (fifteenth century). Appl. Phys. A 2016, 122, 572. [Google Scholar] [CrossRef]
- Brunetto, A.; Bono, G.; Frezzato, F. Er:YAG laser cleaning of ‘San Marziale in Gloria’ by Jacopo Tintoretto in the Church of San Marziale, Venice. J. Inst. Conserv. 2020, 43, 44–58. [Google Scholar] [CrossRef]
- Pouli, P.; Paun, I.-A.; Bounos, G.; Georgiou, S.; Fotakis, C. The potential of UV femtosecond laser ablation for varnish removal in the restoration of painted works of art. Appl. Surf. Sci. 2008, 254, 6875–6879. [Google Scholar] [CrossRef]
- Samorì, C.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Prati, S.; Sciutto, G.; Volpi, F.; Tagliavini, E. The Green Attitude in Art Conservation: Polyhydroxybutyrate–based Gels for the Cleaning of Oil Paintings. ChemistrySelect 2016, 1, 4502–4508. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L.; Weiss, R.G.; Baglioni, P. A new class of gels for the conservation of painted surfaces. J. Cult. Herit. 2008, 9, 386–393. [Google Scholar] [CrossRef]
- Angelova, L.V.; Ormsby, B.; Townsend, J.; Wolbers, R. Gels in the Conservation of Art; Archetype Publications: London, UK, 2017. [Google Scholar]
- Carretti, E.; Grassi, S.; Cossalter, M.; Natali, I.; Caminati, G.; Weiss, R.G.; Baglioni, P.; Dei, L. Poly(vinyl alcohol)−Borate Hydro/Cosolvent Gels: Viscoelastic Properties, Solubilizing Power, and Application to Art Conservation. Langmuir 2009, 25, 8656–8662. [Google Scholar] [CrossRef] [PubMed]
- Carretti, E.; Natali, I.; Matarrese, C.; Bracco, P.; Weiss, R.G.; Baglioni, P.; Salvini, A.; Dei, L. A new family of high viscosity polymeric dispersions for cleaning easel paintings. J. Cult. Herit. 2010, 11, 373–380. [Google Scholar] [CrossRef]
- Lazidou, D.; Teknetzi, I.; Karapanagiotis, I.; Ritzoulis, C.; Panayiotou, C. Poly(vinyl alcohol)-borax films as cleaning agents for icons. Archaeol. Anthropol. Sci. 2019, 11, 6259–6271. [Google Scholar] [CrossRef]
- Duncan, T.T.; Berrie, B.H.; Weiss, R.G. Soft, Peelable Organogels from Partially Hydrolyzed Poly(vinyl acetate) and Benzene-1,4-diboronic Acid: Applications to Clean Works of Art. ACS Appl. Mater. Interfaces 2017, 9, 28069–28078. [Google Scholar] [CrossRef]
- Pensabene Buemi, L.; Petruzzellis, M.L.; Chelazzi, D.; Baglioni, M.; Mastrangelo, R.; Giorgi, R.; Baglioni, P. Twin-chain polymer networks loaded with nanostructured fluids for the selective removal of a non-original varnish from Picasso’s “L’Atelier” at the Peggy Guggenheim Collection, Venice. Herit. Sci. 2020, 8, 77. [Google Scholar] [CrossRef]
- Moskalik-Detalle, A.; Assoun, J.; Joseph, F.; Martiny, M.-L.; Monfort, M. Conservation of murals by Eugène Delacroix at Saint Sulpice, Paris. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 200–208. [Google Scholar]
- Varadinova-Papadaki, S. Gels for removing varnish and surface stains from Bulgarian icons. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 292–293. [Google Scholar]
- Miguirditchian, M.; Engel, N.; Desvois, L.; Capra, A.-L. Cleaning the Adolphe Roger murals at the Church of Notre Dame de Lorette, Paris. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 73–76. [Google Scholar]
- Al-Emam, E.; Motawea, A.G.; Caen, J.; Janssens, K. Soot removal from ancient Egyptian complex painted surfaces using a double network gel: Empirical tests on the ceiling of the sanctuary of Osiris in the temple of Seti I—Abydos. Herit. Sci. 2021, 9, 1. [Google Scholar] [CrossRef]
- Al-Emam, E.; Motawea, A.G.; Janssens, K.; Caen, J. Evaluation of polyvinyl alcohol–borax/agarose (PVA–B/AG) blend hydrogels for removal of deteriorated consolidants from ancient Egyptian wall paintings. Herit. Sci. 2019, 7, 22. [Google Scholar] [CrossRef]
- Carson, T.; Cerrito, J. New Catholic Encyclopedia. 2, 2; Thomson/Gale in Association with the Catholic University of America: Detroit, MI, USA; London, UK, 2003. [Google Scholar]
- Tosf, H.; Church, C. Manual of the St. John Berchmans Sanctuary Society: With a Sketch of the Saint’s Life; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2016. [Google Scholar]
- Prims, F. Op de Gronden van Sint-Jan-Berchmans College: Van Houtmere tot Carmelkloster, stapelhuis en Gymnasium; Sint-Jan Berchmans College: Antwertp, Belgium, 1949. [Google Scholar]
- Kunsten, K.M.v.S.; Buyck, J. Catalogus Schilderijen 19de en 20ste Eeuw; Ministerie van Nederlandse Cultuur, Koninklijk Museum voor Schone Kunsten: Antwertp, Belgium, 1977. [Google Scholar]
- Nauts, H. Catalogus Stedelijk Kunstbezit Sint-Niklaas; Staatsbestuur van Sint-Niklaas: Sint-Niklaas, Belgium, 1988. [Google Scholar]
- Dotremont, G.; Martiny, V.G. De Stichting Godecharle, 1871–1971; Provinciale Commissie voor Studiebeurzenstichtingen van Brabant: Brussels, Belgium, 1971. [Google Scholar]
- Coenjaerts, P.; Winckelmans, P. Sint-Jan Berchmans: Een Eeuw Collegeleven; Sint-Jan Berchmanscollege: Antwerp, Belgium, 1989. [Google Scholar]
- Yang, X.; Ji, X.; Cao, Y.; Yu, T. Studies on wall painting materials and techniques at two historic buildings in Gyantse, Tibet. Herit. Sci. 2019, 7, 40. [Google Scholar] [CrossRef]
- Schmidt, B.A.; Ziemann, M.A.; Pentzien, S.; Gabsch, T.; Koch, W.; Krüger, J. Technical analysis of a Central Asian wall painting detached from a Buddhist cave temple on the northern Silk Road. Stud. Conserv. 2016, 61, 113–122. [Google Scholar] [CrossRef] [Green Version]
- de la Rie, E.R. Fluorescence of paint and varnish layers (Part 1). Stud. Conserv. 1982, 27, 1–7. [Google Scholar] [CrossRef]
- de Viguerie, L.; Payard, P.A.; Portero, E.; Walter, P.; Cotte, M. The drying of linseed oil investigated by Fourier transform infrared spectroscopy: Historical recipes and influence of lead compounds. Prog. Org. Coat. 2016, 93, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Gabrieli, F.; Rosi, F.; Vichi, A.; Cartechini, L.; Pensabene Buemi, L.; Kazarian, S.G.; Miliani, C. Revealing the Nature and Distribution of Metal Carboxylates in Jackson Pollock’s Alchemy (1947) by Micro-Attenuated Total Reflection FT-IR Spectroscopic Imaging. Anal. Chem. 2017, 89, 1283–1289. [Google Scholar] [CrossRef]
- Beltran, V.; Salvadó, N.; Butí, S.; Cinque, G. Micro infrared spectroscopy discrimination capability of compounds in complex matrices of thin layers in real sample coatings from artworks. Microchem. J. 2015, 118, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Siidra, O.; Nekrasova, D.; Depmeier, W.; Chukanov, N.; Zaitsev, A.; Turner, R. Hydrocerussite-related minerals and materials: Structural principles, chemical variations and infrared spectroscopy. Acta Crystallogr. Sect. B 2018, 74, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Legodi, M.A.; de Waal, D.; Potgieter, J.H.; Potgieter, S.S. Rapid determination of CaCO3 in mixtures utilising FT—IR spectroscopy. Miner. Eng. 2001, 14, 1107–1111. [Google Scholar] [CrossRef]
- Cather, S.; Howard, H. The use of wax and wax-resin preservatives on English mediaeval wall paintings: Rationale and consequences. Stud. Conserv. 1986, 31, 48–53. [Google Scholar] [CrossRef]
- Beckett, B. The search for an enduring painting technique: Franz Fernbach and his encaustic technique as a restoration procedure for wall-paintings in the early nineteenth century. In Conservation in the Nineteenth Century; Brajer, I., Ed.; Archetype Publications: London, UK, 2013; pp. 105–115. [Google Scholar]
- Ford, T.; Rizzo, A.; Hendriks, E.; Frøysaker, T.; Caruso, F. A non-invasive screening study of varnishes applied to three paintings by Edvard Munch using portable diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). Herit. Sci. 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Azémard, C.; Vieillescazes, C.; Ménager, M. Effect of photodegradation on the identification of natural varnishes by FT-IR spectroscopy. Microchem. J. 2014, 112, 137–149. [Google Scholar] [CrossRef]
- The Infrared and Raman Users Group (IRUG). Available online: http://www.irug.org/ (accessed on 6 May 2019).
- Learner, T. Analysis of Modern Paints; The Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Miller, E. The Nebamun Wall Paintings of the British Museum. In The Conservation of Decorated Surfaces on Earthen Architecture: Proceedings from the International Colloquium Organized by the Getty Conservation Institute and the National Park Service Mesa Verde National Park, Colorado, USA 22–25 September 2004; Rainer, L., Rivera, A.B., Eds.; The Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Bandaranayake, S. The Dambulla Rock Temple Complex, Sri Lanka: Ten years of management, research, and conservation. In Conservation of Ancient Sites on the Silk Road: Proceedings of an International Conference on the Conservation of Grotto Sites, Mogao Grottoes, Dunhuang, China, 3–8 October 1993; Agnew, N., Ed.; Getty Conservation Institute: Los Angeles, CA, USA, 1997. [Google Scholar]
- Phenix, A.; Wolbers, R. Removal of varnish: Organic solvents as cleaning agents. In The Conservation of Easel Paintings; Stoner, J.H., Rushfield, R., Eds.; Routledge: London, UK, 2012; pp. 524–555. [Google Scholar]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Al-Emam, E.; Soenen, H.; Caen, J.; Janssens, K. Characterization of polyvinyl alcohol-borax/agarose (PVA-B/AG) double network hydrogel utilized for the cleaning of works of art. Herit. Sci. 2020, 8, 106. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Emam, E.; Beltran, V.; De Meyer, S.; Nuyts, G.; Wetemans, V.; De Wael, K.; Caen, J.; Janssens, K. Removal of a Past Varnish Treatment from a 19th-Century Belgian Wall Painting by Means of a Solvent-Loaded Double Network Hydrogel. Polymers 2021, 13, 2651. https://doi.org/10.3390/polym13162651
Al-Emam E, Beltran V, De Meyer S, Nuyts G, Wetemans V, De Wael K, Caen J, Janssens K. Removal of a Past Varnish Treatment from a 19th-Century Belgian Wall Painting by Means of a Solvent-Loaded Double Network Hydrogel. Polymers. 2021; 13(16):2651. https://doi.org/10.3390/polym13162651
Chicago/Turabian StyleAl-Emam, Ehab, Victoria Beltran, Steven De Meyer, Gert Nuyts, Vera Wetemans, Karolien De Wael, Joost Caen, and Koen Janssens. 2021. "Removal of a Past Varnish Treatment from a 19th-Century Belgian Wall Painting by Means of a Solvent-Loaded Double Network Hydrogel" Polymers 13, no. 16: 2651. https://doi.org/10.3390/polym13162651