Artificial Light at Night Advances Spring Phenology in the United States
Abstract
:1. Introduction
- (1)
- How is spring phenology affected by ALAN and in relation to other phenology cues?
- (2)
- Does the impact on spring phenology vary spatially and, if so, what are the underlying mechanisms explaining the spatial pattern?
- (3)
- How does the ALAN’s impact in urban areas differ from that in natural ecosystems?
2. Materials and Methods
2.1. Datesets and Preprocessing
2.1.1. Satellite-Based Spring Phenology Data
2.1.2. Nighttime Light Images
- (1)
- Visible Infrared Imaging Radiometer Suite (VIIRS, 2012–2018) nighttime light images from Suomi-NPP satellite. VIIRS, a new generation of NTL sensor (2012) with an on-board calibration system, provides 500-m monthly NTL images in consistent and non-saturated radiance values [38,39]. We resampled the VIIRS images into 1-km resolution and aggregated them into annually averaged NTL images from 2012 to 2018.
- (2)
- Long-term NTL time series from harmonized DMSP-OLS and VIIRS data (DMSP-VIIRS, 2001–2018) [40]. The DMSP-VIIRS data provides annually averaged NTL with 1-km spatial resolution from 2001–2018. This harmonized NTL data reduces the cross-sensor difference between DMSP-OLS and VIIRS, expanding the temporal range of NTL time series. Note that the NTL intensity of the harmonized NTL data is still the relative NTL intensity.
2.1.3. Climate Data
2.1.4. Land Cover Maps
2.2. Analysis
3. Results
3.1. Phenological Impact of ALAN
- (1)
- Data availability. Subjected to the availability of NTL data and the corresponding phenology and climate data, VIIRS data were only available for 6.12 ± 1.17 yr. for each pixel, while DMSP-OLS data is available for 12.2 ± 3.28 yr. The partial correlation analysis was vulnerable to the impact of short-term observations [53].
- (2)
- Overpass time. DMSP-OLS (7:30 p.m.) has a more ideal overpass time than VIIRS (1:30 a.m.) because the peak lighting hour is approximately from sunset to 22:00 p.m. [54]. Using DMSP-OLS data enables us to evaluate the phenological response to ALAN when most artificial lights are on.
- (3)
- Despite a longer temporal coverage of DMSP-VIIRS data, the uncertainties of cross-sensor calibration algorithm to generate DMSP-VIIRS data may propagate to and confound our analysis [55].
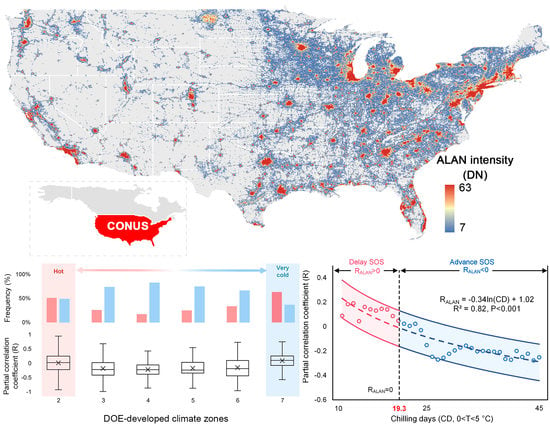
3.2. Divergent Spatial Pattern of the Phenological Impact of ALAN
3.3. Urban. vs. Non-Urban. Area
4. Discussions
4.1. Mechanisms of the Divergent Spatial Pattern
4.1.1. The Interplay among Chilling, Forcing and Photoperiod
4.1.2. Chilling Insufficient
4.1.3. Differences in Species’ Life Strategies
4.2. Implications
- (1)
- the interplay among forcing, chilling and photoperiod;
- (2)
- chilling insufficiency, as we found the phenological impact of ALAN was sensitive to the accumulated chilling days;
- (3)
- the differences in life strategies of species. The results have an implication to understand the interaction mechanism between phenology shifts and environmental stimuli. It helps to better forecast how phenology will be influenced by and feedback to the warming climate and the environment being modified by human activities. Revealing the spatial variation of the impact of ALAN and its linkage to other environmental cues of phenology is a complement to in situ observation and experiment-based studies. Our results imply that previous studies about daylength and photoperiod may underestimate its actual potential in shifting phenology, especially for places where ALAN is prevailing. Hence, the long-lasting controversy on how ALAN (or photoperiod) affects phenology can be a result of not taking ALAN into consideration (for studies related to photoperiod) and/or the divergent spatial pattern of the ALAN-SOS relationship (for studies related to photoperiod and ALAN). We foresee a promising potential in improving the existing temperature-photoperiod based phenology models by incorporating the impact of ALAN [66].
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agr. Forest Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Cook, B.I.; Wolkovich, E.M.; Parmesan, C. Divergent responses to spring and winter warming drive community level flowering trends. Proc. Natl. Acad. Sci. USA 2012, 109, 9000–9005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.g.; Briede, A. European phenological response to climate change matches the warming pattern. Global Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Silander, J.A., Jr. Deciduous forest responses to temperature, precipitation, and drought imply complex climate change impacts. Proc. Natl. Acad. Sci. USA 2015, 112, 13585–13590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zohner, C.M.; Benito, B.M.; Svenning, J.-C.; Renner, S.S. Day length unlikely to constrain climate-driven shifts in leaf-out times of northern woody plants. Nature Clim. Chang. 2016, 6, 1120–1123. [Google Scholar] [CrossRef]
- Penuelas, J.; Rutishauser, T.; Filella, I. Ecology. Phenology feedbacks on climate change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korner, C.; Basler, D. Plant science. Phenology under global warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Laube, J.; Sparks, T.H.; Estrella, N.; Hofler, J.; Ankerst, D.P.; Menzel, A. Chilling outweighs photoperiod in preventing precocious spring development. Global Chang. Biol. 2014, 20, 170–182. [Google Scholar] [CrossRef]
- Way, D.A.; Montgomery, R.A. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant. Cell Environ. 2015, 38, 1725–1736. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Wolkovich, E.M. Temperature and photoperiod drive spring phenology across all species in a temperate forest community. New Phytol. 2018, 219, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, D.; Tidau, S.; Jenkins, S.; Davies, T. Artificial skyglow disrupts celestial migration at night. Curr. Biol. 2020, 30, R696–R697. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J. Lighting up the nighttime. Science 2018, 362, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Weng, Q.; Huang, L.; Wang, K.; Deng, J.; Jiang, R.; Ye, Z.; Gan, M. A new source of multi-spectral high spatial resolution night-time light imagery—JL1-3B. Remote Sens. Environ. 2018, 215, 300–312. [Google Scholar] [CrossRef]
- Aubé, M.; Kocifaj, M.; Zamorano, J.; Lamphar, H.S.; de Miguel, A.S. The spectral amplification effect of clouds to the night sky radiance in Madrid. J. of Quant. Spectrosc. Radiat. Transf. 2016, 181, 11–23. [Google Scholar] [CrossRef]
- Jiang, W.; He, G.; Long, T.; Wang, C.; Ni, Y.; Ma, R. Assessing Light Pollution in China Based on Nighttime Light Imagery. Remote Sens. 2017, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennie, J.; Davies, T.W.; Cruse, D.; Bell, F.; Gaston, K.J. Artificial light at night alters grassland vegetation species composition and phenology. J. Appl. Ecol. 2018, 55, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Ayalon, I.; de Barros Marangoni, L.F.; Benichou, J.I.C.; Avisar, D.; Levy, O. Red Sea corals under Artificial Light Pollution at Night (ALAN) undergo oxidative stress and photosynthetic impairment. Global Chang. Biol. 2019, 25, 4194–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennie, J.; Davies, T.W.; Cruse, D.; Gaston, K.J. Ecological effects of artificial light at night on wild plants. J. Ecol. 2016, 104, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.J.; Duffy, J.P.; Gaston, S.; Bennie, J.; Davies, T.W. Human alteration of natural light cycles: Causes and ecological consequences. Oecologia 2014, 176, 917–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubé, M.; Marseille, C.; Farkouh, A.; Dufour, A.; Simoneau, A.; Zamorano, J.; Roby, J.; Tapia, C. Mapping the Melatonin Suppression, Star Light and Induced Photosynthesis Indices with the LANcube. Remote Sens. 2020, 12, 3954. [Google Scholar] [CrossRef]
- Xue, X.; Lin, Y.; Zheng, Q.; Wang, K.; Zhang, J.; Deng, J.; Abubakar, G.A.; Gan, M. Mapping the fine-scale spatial pattern of artificial light pollution at night in urban environments from the perspective of bird habitats. Sci. Total Environ. 2020, 702. [Google Scholar] [CrossRef] [PubMed]
- Škvareninová, J.; Tuhárska, M.; Škvarenina, J.; Babálová, D.; Slobodníková, L.; Slobodník, B.; Středová, H.; Minďaš, J. Effects of light pollution on tree phenology in the urban environment. Morav. Geogr. Rep. 2017, 25, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Massetti, L. Assessing the impact of street lighting on Platanus x acerifolia phenology. Urban. For. Urban. Gree. 2018, 34, 71–77. [Google Scholar] [CrossRef]
- Briggs, W.R. Physiology of plant responses to artificial lighting. Ecol. Conseq. Artif. Night Lighting 2006, 389–411. [Google Scholar]
- Chen, G.; Li, X.; Liu, X.; Chen, Y.; Liang, X.; Leng, J.; Xu, X.; Liao, W.; Qiu, Y.a.; Wu, Q.; et al. Global projections of future urban land expansion under shared socioeconomic pathways. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, H.; Bai, X.; Brondizio, E.S.; Lwasa, S. The urban south and the predicament of global sustainability. Nat. Sustain. 2018, 1, 341–349. [Google Scholar] [CrossRef]
- Gray, J.; Sulla-Menashe, D.; Friedl, M.A. User Guide to Collection 6 MODIS Land Cover Dynamics (MCD12Q2) Product; NASA EOSDIS Land Processes DAAC: Missoula, MT, USA, 2019. [Google Scholar]
- Meng, L.; Zhou, Y.; Li, X.; Asrar, G.R.; Mao, J.; Wanamaker, A.D.; Wang, Y. Divergent responses of spring phenology to daytime and nighttime warming. Agr. Forest Meteorol. 2020, 281. [Google Scholar] [CrossRef]
- Cong, N.; Wang, T.; Nan, H.; Ma, Y.; Wang, X.; Myneni, R.B.; Piao, S. Changes in satellite-derived spring vegetation green-up date and its linkage to climate in China from 1982 to 2010: A multimethod analysis. Global Chang. Biol. 2013, 19, 881–891. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, Y.H.; Zeng, Z.; Huang, M.; Li, X.; Piao, S. Temperature, precipitation, and insolation effects on autumn vegetation phenology in temperate China. Global Chang. Biol. 2016, 22, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Seto, K.C. Mapping urbanization dynamics at regional and global scales using multi-temporal DMSP/OLS nighttime light data. Remote Sens. Environ. 2011, 115, 2320–2329. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Ziskin, D.; Baugh, K.E.; Tuttle, B.T.; Ghosh, T.; Pack, D.W.; Erwin, E.H.; Zhizhin, M. A Fifteen Year Record of Global Natural Gas Flaring Derived from Satellite Data. Energies 2009, 2, 595–622. [Google Scholar] [CrossRef]
- Liu, Z.; He, C.; Zhang, Q.; Huang, Q.; Yang, Y. Extracting the dynamics of urban expansion in China using DMSP-OLS nighttime light data from 1992 to 2008. Landsc. Urban. Plan. 2012, 106, 62–72. [Google Scholar] [CrossRef]
- Zheng, Q.; Weng, Q.; Wang, K. Correcting the Pixel Blooming Effect (PiBE) of DMSP-OLS nighttime light imagery. Remote Sens. Environ. 2020, 240. [Google Scholar] [CrossRef]
- Sanchez de Miguel, A.; Kyba, C.C.M.; Zamorano, J.; Gallego, J.; Gaston, K.J. The nature of the diffuse light near cities detected in nighttime satellite imagery. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Levin, N.; Kyba, C.C.M.; Zhang, Q.; de Miguel, A.S.; Roman, M.O.; Li, X.; Portnov, B.A.; Molthan, A.L.; Jechow, A.; Miller, S.D.; et al. Remote sensing of night lights: A review and an outlook for the future. Remote Sens. Environ. 2020, 237. [Google Scholar] [CrossRef]
- Zheng, Q.; Weng, Q.; Wang, K. Characterizing urban land changes of 30 global megacities using nighttime light time series stacks. ISPRS J. Photogramm. Remote Sens. 2021, 173, 10–23. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhao, M.; Zhao, X. A harmonized global nighttime light dataset 1992–2018. Scientific data 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Román, M.O.; Wang, Z.; Sun, Q.; Kalb, V.; Miller, S.D.; Molthan, A.; Schultz, L.; Bell, J.; Stokes, E.C.; Pandey, B.; et al. NASA’s Black Marble nighttime lights product suite. Remote Sens. Environ. 2018, 210, 113–143. [Google Scholar] [CrossRef]
- Smith, H. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Wang, H.; Ciais, P.; Peñuelas, J.; Myneni, R.B.; Desai, A.R.; Gough, C.M.; Gonsamo, A.; Black, A.T.; et al. Contrasting responses of autumn-leaf senescence to daytime and night-time warming. Nat. Clim. Chang. 2018, 8, 1092–1096. [Google Scholar] [CrossRef] [Green Version]
- Sulla-Menashe, D.; Gray, J.M.; Abercrombie, S.P.; Friedl, M.A. Hierarchical mapping of annual global land cover 2001 to present: The MODIS Collection 6 Land Cover product. Remote Sens. Environ. 2019, 222, 183–194. [Google Scholar] [CrossRef]
- Piao, S.; Tan, J.; Chen, A.; Fu, Y.H.; Ciais, P.; Liu, Q.; Janssens, I.A.; Vicca, S.; Zeng, Z.; Jeong, S.J.; et al. Leaf onset in the northern hemisphere triggered by daytime temperature. Nat. Commun. 2015, 6, 6911. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.H.; Zhang, X.; Piao, S.; Hao, F.; Geng, X.; Vitasse, Y.; Zohner, C.; Penuelas, J.; Janssens, I.A. Daylength helps temperate deciduous trees to leaf-out at the optimal time. Global Chang. Biol. 2019, 25, 2410–2418. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Penuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Global Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Somers-Yeates, R.; Bennie, J.; Economou, T.; Hodgson, D.; Spalding, A.; McGregor, P.K. Light pollution is associated with earlier tree budburst across the United Kingdom. Proc. Biol. Sci. 2016, 283. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. Hortscience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Whitman, C.M.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Lamp type and irradiance level for daylength extensions influence flowering of Campanula carpaticaBlue Clips’, Coreopsis grandifloraEarly Sunrise’, and Coreopsis verticillataMoonbeam’. J. Am. Soc. Hortic. Sci. 1998, 123, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Kyba, C.C.M.; Kuester, T.; Sanchez de Miguel, A.; Baugh, K.; Jechow, A.; Holker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, e1701528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiao, J.; Li, X.; Cheng, G.; Ma, M.; Zhu, G.; Altaf Arain, M.; Andrew Black, T.; Jassal, R.S. No trends in spring and autumn phenology during the global warming hiatus. Nat. Commun. 2019, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- Elvidge, C.; Baugh, K.; Zhizhin, M.; Hsu, F.-C. Why VIIRS data are superior to DMSP for mapping nighttime lights. Proc. Asia Pac. Adv. Netw 2013, 35, 62. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Weng, Q.; Wang, K. Developing a new cross-sensor calibration model for DMSP-OLS and Suomi-NPP VIIRS night-light imageries. ISPRS J. Photogramm. Remote Sens. 2019, 153, 36–47. [Google Scholar] [CrossRef]
- Burleyson, C. 2012 IECC Climate Zones; Pacific Northwest National Laboratory 2; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2020. [Google Scholar]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol Rev. Camb Philos Soc. 2013, 88, 912–927. [Google Scholar] [CrossRef]
- Neff, M.M.; Fankhauser, C.; Chory, J. Light: An indicator of time and place. Gene Dev. 2000, 14, 257–271. [Google Scholar]
- Basler, D.; Körner, C. Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agr. Forest Meteorol. 2012, 165, 73–81. [Google Scholar] [CrossRef]
- Kocifaj, M.; Solano-Lamphar, H.A.; Videen, G. Night-sky radiometry can revolutionize the characterization of light-pollution sources globally. Proc. Natl. Acad. Sci. USA 2019, 116, 7712–7717. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Piao, S.; Ciais, P.; Li, J.; Friedlingstein, P.; Koven, C.; Chen, A. Spring temperature change and its implication in the change of vegetation growth in North America from 1982 to 2006. Proc. Natl. Acad. Sci. USA 2011, 108, 1240–1245. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.D.; Bailey, A.S.; Denny, E.G.; Martin, C.W.; O’Keefe, J. Phenology of a northern hardwood forest canopy. Global Chang. Biol. 2006, 12, 1174–1188. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Furze, M.E.; Seyednasrollah, B.; Krassovski, M.B.; Latimer, J.M.; Nettles, W.R.; Heiderman, R.R.; et al. Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature 2018, 560, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, A.; Donnelly, A. The ecological significance of phenology in four different tree species: Effects of light and temperature on bud burst. Int. J. Biometeorol. 2011, 55, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Tiffney, B.; Manchester, S. The influence of physical environment on phytogeographic continuity and phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant. Sci. 2001, 162, S3–S17. [Google Scholar] [CrossRef]
- Migliavacca, M.; Sonnentag, O.; Keenan, T.F.; Cescatti, A.; O’Keefe, J.; Richardson, A.D. On the uncertainty of phenological responses to climate change, and implications for a terrestrial biosphere model. Biogeosciences 2012, 9, 2063–2083. [Google Scholar] [CrossRef] [Green Version]
- Elvidge, C.D.; Cinzano, P.; Pettit, D.R.; Arvesen, J.; Sutton, P.; Small, C.; Nemani, R.; Longcore, T.; Rich, C.; Safran, J.; et al. The Nightsat mission concept. Int. J. Remote Sens. 2007, 28, 2645–2670. [Google Scholar] [CrossRef]
- Li, X.; Levin, N.; Xie, J.; Li, D. Monitoring hourly night-time light by an unmanned aerial vehicle and its implications to satellite remote sensing. Remote Sens. Environ. 2020, 247. [Google Scholar] [CrossRef]
- Peaucelle, M.; Janssens, I.A.; Stocker, B.D.; Descals Ferrando, A.; Fu, Y.H.; Molowny-Horas, R.; Ciais, P.; Penuelas, J. Spatial variance of spring phenology in temperate deciduous forests is constrained by background climatic conditions. Nat. Commun. 2019, 10, 5388. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wu, C.; Ciais, P.; Penuelas, J.; Dai, J.; Fu, Y.; Ge, Q. Overestimation of the effect of climatic warming on spring phenology due to misrepresentation of chilling. Nat. Commun. 2020, 11, 4945. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Teo, H.C.; Koh, L.P. Artificial Light at Night Advances Spring Phenology in the United States. Remote Sens. 2021, 13, 399. https://doi.org/10.3390/rs13030399
Zheng Q, Teo HC, Koh LP. Artificial Light at Night Advances Spring Phenology in the United States. Remote Sensing. 2021; 13(3):399. https://doi.org/10.3390/rs13030399
Chicago/Turabian StyleZheng, Qiming, Hoong Chen Teo, and Lian Pin Koh. 2021. "Artificial Light at Night Advances Spring Phenology in the United States" Remote Sensing 13, no. 3: 399. https://doi.org/10.3390/rs13030399