Abstract
Artificial lighting at night (ALAN) produced by urban, industrial, and roadway lighting, as well as other sources, has dramatically increased in recent decades, especially in coastal environments that support dense human populations. Artificial “lightscapes” are characterized by distinct spatial, temporal, and spectral patterns that can alter natural patterns of light and dark with consequences across levels of biological organization. At the individual level, ALAN can elicit a suite of physiological and behavioral responses associated with light-mediated processes such as diel activity patterns and predator-prey interactions. ALAN has also been shown to modify community composition and trophic structure, with implications for ecosystem-level processes including primary productivity, nutrient cycling, and the energetic linkages between aquatic and terrestrial systems. Here, we review the state of the science relative to the impacts of ALAN on estuaries, which is an important step in assessing the long-term sustainability of coastal regions. We first consider how multiple properties of ALAN (e.g., intensity and spectral content) influence the interaction between physiology and behavior of individual estuarine biota (drawing from studies on invertebrates, fishes, and birds). Second, we link individual- to community- and ecosystem-level responses, with a focus on the impacts of ALAN on food webs and implications for estuarine ecosystem functions. Coastal aquatic communities and ecosystems have been identified as a key priority for ALAN research, and a cohesive research framework will be critical for understanding and mitigating ecological consequences.
Similar content being viewed by others
Introduction
Artificial lighting at night (ALAN) alters natural patterns of light and darkness by introducing light that varies in intensity and spectral composition during typically dark periods (Gaston et al. 2013). Use of, and technological advances in, electric lighting have rapidly expanded since the incandescent lightbulbs of the early 1900s; ALAN now generates levels of intensity visible in satellite imagery of the Earth’s surface at night. Life has evolved under dynamic environmental conditions, but changes to the daily and seasonal light regimes associated with ALAN present a truly novel ecological stressor. An expanding avenue of research has documented the ecological impacts of down-welling (direct) ALAN from urban, commercial, and industrial sources, increasing the recognition of light pollution as a global environmental concern (Longcore and Rich 2004, Rich and Longcore 2006, RCEP 2009, Davies and Smyth 2018, Sanders and Gaston 2018).
Light acts as a vital resource for aquatic and terrestrial organisms by driving primary production, informing visual perception, and maintaining biological rhythms (Navara and Nelson 2007, Kronfeld-Schor et al. 2013, Ouyang et al. 2018). Research based in both aquatic and terrestrial ecosystems has shown that ALAN can profoundly influence biological systems from the cellular-to-ecosystem levels. Ecological impacts include changes in daily activity patterns (e.g., foraging, Bird et al. 2004, Dwyer et al. 2013, Dominoni et al. 2014), habitat use (Henn et al. 2014), community organization (Davies et al. 2012, Becker and Suthers 2014), and provisioning of ecosystem services (Lyytimäki 2013, Lewanzik and Voigt 2014). In urban settings, ALAN can cross aquatic-terrestrial ecosystem boundaries as light permeates through riparian habitats (Perkin et al. 2011). For example, in a study investigating cross-habitat impacts of ALAN, Meyer and Sullivan (2013) found that elevated ALAN levels were associated with reduced diversity, body size, and biomass of emerging aquatic insects, which in turn are expected to have implications for terrestrial consumers (e.g., spiders, birds, bats) that rely on aquatic prey subsidies (reviewed in Baxter et al. 2005). However, few studies have explicitly tested for direct and indirect consequences of ALAN across aquatic-terrestrial boundaries (but see Manfrin 2017, Sullivan et al. in press).
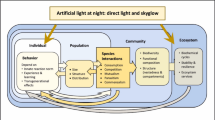
One of the key knowledge gaps in our understanding of the effects of ALAN is how responses scale from the individual organism to the functioning of ecosystems. For example, if a fish that is normally active during the day starts foraging at night under ALAN, there will be consequences for its prey. What effect might this alteration in behavior of one organism have on species interactions, community dynamics, and ultimately the functioning of the ecosystem? Coastal-estuarine ecosystems, which are disproportionately affected by ALAN due to urbanization along coastal waterways (Aubrecht et al. 2010a, Davies et al. 2016), may offer insights on individual-to-ecosystem-level effects of ALAN. Aquatic and terrestrial ecosystems are known to be tightly linked in estuaries and coastal regions. Thus, further investigation using these linked systems—and what we know of organismal, community, and ecosystem level responses to ALAN—offer an opportunity to make connections across levels of biological organization (Fig. 1).
Conceptual map of individual- to ecosystem-level responses to ALAN in estuarine ecosystems with summary of responses and example mechanisms for responses at each level of biological organization. Highlighted frames (communities, food webs, ecosystems) represent levels of organization whose response to ALAN is currently underrepresented in the literature. Quantifying and understanding linkages across levels of biological organization (e.g., individuals-ecosystem linkages/feedbacks) will be crucial in developing an integrative ALAN research and conservation framework. This conceptual map serves as the basis for a cohesive research framework and key research questions (see Conclusions & Future Directions)
Estuaries and coastal wetlands provide vital ecosystem services by contributing to fisheries, water quality, carbon sequestration, coastal protection, and pollution control (Levin et al. 2001, Barbier et al. 2011). The introduction of ALAN poses a growing threat to estuarine biodiversity and ecosystem services in densely populated coastal habitats (Davies et al. 2014, Stanley et al. 2015). Assessments of ALAN in coastal environments are limited (Depledge et al. 2010), yet coastal-estuarine ecosystems are often replete with lighting sources including urban centers, off-shore oil and fisheries operations, commercial and recreational vessels, and the scattering of light in the atmosphere and downcasting by clouds (Kyba et al. 2011). ALAN now infiltrates many protected coastal areas including mangrove forests in subtropical and tropical regions (Aubrecht et al. 2010b, Bennie et al. 2015b). Furthermore, global emissions of ALAN are projected to increase by ~ 6% each year (Hölker et al. 2010a), with ecological impacts to coastal marine and estuarine ecosystems. Challenges to managing the valuable biological resources of these regions considering this and many other environmental stressors in estuaries should be met with a strong understanding of system-specific processes and ecological impacts.
Here, we synthesize known and potential impacts of ALAN on estuarine organisms, communities, and ecosystem functioning (Fig. 1), with the goal of highlighting linkages between levels of biological organization that require more attention. We draw from studies that focus on physiology, behavior, and ecology of aquatic-associated animals and on the ecological effects of natural and artificial light in terrestrial, freshwater, estuarine, and marine systems. First, we consider how the intensity, spectral composition, and duration of ALAN impacts the physical light environment at night, changing interactions between the visual physiology and behavior of estuarine biota at the individual level. We then discuss potential links from individual-level responses to community and ecosystem responses, with a focus on the impacts of ALAN on ecological trophic networks (i.e., food webs). Finally, we discuss implications for estuarine ecosystem functioning and the ways that understanding individual-to-ecosystem linkages can inform future research and conservation efforts.
ALAN and the Light Environment
Understanding how ALAN affects the natural light-dark regime and how animals perceive these changes is fundamental to linking individual and population responses to ALAN with community and ecosystem processes. Sanders and Gaston (2018) categorize light (and the absence of light) based on how an organism uses light: (i) as a resource, and (ii) as a source of information. Light as a resource contributes to photosynthesis and primary production in the aquatic environment (reviewed in Kirk 2011). Animals perceive information from light in various ways, which requires light-sensitive photoreceptors. The detection and neural processing of variability in the light environment can inform scotopic (dim-light) and photopic (color) vision, spatial orientation, and biological rhythms (including circadian, migratory, and reproductive cycles). In this section, we provide an overview of light in the environment, how it is detected and used by animals with an emphasis on estuarine communities, and how ALAN alters the natural lightscape. We briefly outline how animals detect light and then offer several relevant examples of ways in which animals utilize light as an information source.
The Estuarine Lightscape
Light environments are determined by the cyclical nature of light intensity and spectral patterns and by the way that light moves through air and water. The timing and duration of light-dark cycles throughout the day and year have been predictable cues for organisms through evolutionary time, until the last century when ALAN was introduced. The predictable nature of the transition from light to dark on daily and seasonal time scales has favored the evolution of light-detection mechanisms coupled with behavioral and physiological shifts (e.g., diel migration in invertebrates [zooplankton, Moore et al. 1998], melatonin production and decreased activity in diurnal fish [Ouyang et al. 2018]). However, at a given time and environment, the nature of light is largely determined by the absorption and scattering of down-welling light moving through the medium (e.g., air or water), and light reflecting from a surface (Lythgoe 1979). This dynamic of predictable light cycles and variation in the intensity and color of light at a given time and place creates complex lightscapes with which organisms must contend.
Light at the air-water interface is critical in linking terrestrial and aquatic biota. In air, light is scattered and absorbed by particulates ranging from water molecules to dust particles, resulting in variable sky colors (Lythgoe 1979). Structurally complex terrestrial environments, such as forested riparian zones, further filter certain wavelengths of light so that the amount and color of light reaching the water surface is much reduced (Endler 1993). Light is then either reflected into the air or penetrates the water surface and is refracted. Animals living and foraging at the air-water interface exhibit numerous adaptations to this complex lighting environment. For example, wading birds must have sufficient visual acuity to find their prey through this air-water interface, while avoiding being detected by their fish prey looking up through the interface. Green and Leberg (2005) found that white plumage color in snowy egret (Egretta thula) and little blue heron (E. caerulea) was more cryptic than dark plumage in open intertidal zones based on the response of their preferred prey (crayfish, Procambarus spp., western mosquitofish, Gambusia affinis). This advantage disappeared when the birds were viewed against a vegetated background.
Underwater, selective absorption of wavelengths by suspended particulates influences irradiance (i.e., radiant flux received by a surface per unit area) at different water depths and depending on particulate shape and size (Loew and McFarland 1990). In shallow coastal waters, the photic environment is dominated by medium-to-short wavelengths of light, producing the blue-green color typical of these zones. In organic-rich brackish and freshwaters, dissolved organic carbon (DOC) creates a red-shifted (dominated by longer wavelengths) and less-intense (darker) photic environment (Lythgoe 1979). Additionally, turbidity derived from suspended silt, phytoplankton, detritus, and other particulate and dissolved materials (e.g., colored dissolved organic matter [CDOM]) determines the spectral absorption and scattering properties of coastal surface waters (Cannizzaro et al. 2013). Spectral absorption by phytoplankton and detritus can determine the light field of aquatic habitats just as a forest canopy filters light that permeates into the understory (Endler 1990). For example, in the subtropical Pearl River estuary of China, spectral absorbance by non-algal particulates was more important than within the inner river plume, where terrestrial detritus from runoff dominates the visual scene (Cao et al. 2003); and further, algal particulate absorption was found to be more important to spectral absorbance in more saline coastal habitats. Similarly, in a subtropical Florida estuary, shorter wavelength ultraviolet (UV) light is more readily absorbed by CDOM in the upper estuary compared to downstream (Chen et al. 2015), contributing to a red-shifted light environment in the upper estuary. Light attenuation related to this turbidity “filter” can further drive the distribution and productivity of phytoplankton, benthic algae, macrophytes (Burford et al. 2012, Cloern et al. 2014, Radabaugh et al. 2014), and potentially higher trophic-level consumers.
Light as Information
Given the predictable nature of light cycles, animals have evolved a variety of mechanisms for detecting and interpreting variation in the intensity (and in many cases, color) and direction of light. Key to light detection are photoreceptors, which can be found in the retinae of animal eyes, but also in the integument and internal organs (e.g., pineal gland of non-mammalian vertebrates). Evolutionary adaptations in visual physiology—spectral sensitivity, visual orientation, and circadian functioning—are examples of how ambient light can function as a selective pressure for estuarine animals. By changing the color (or spectral qualities), duration, and relative orientation of down-welling and polarized light in the nocturnal environment, ALAN is expected to disrupt these basic mechanisms and natural ecological functioning in estuaries.
Light Detection
Animal visual systems are composed of light-sensitive progenitor cells and photoreceptors that evolved in response to the spectral qualities of the photic environment (Lythgoe 1979). As such, the ability to detect different light intensities and wavelengths varies among individuals and species that inhabit distinct light environments (Cronin et al. 2014). For example, freshwater threespine stickleback (Gasterosteus aculeatus) exhibited differences in visual sensitivity important for mate selection in clear versus tannin-stained lakes (Boughman 2001). Studying the spectral qualities of terrestrial light environments has also informed our understanding of diurnal visual ecology, and specifically how color vision is integral in detection of food and prey resources, mates and competitors, and potential threats (Endler 1993). Spatial and temporal niche partitioning have led to the evolution of visual sensory systems in invertebrates, birds, and fishes that are specialized for performance in different light environments (e.g., habitat or time of day; Cronin et al. 2014). For example, the eyes of nocturnal fish species are generally characterized by a larger lens and pupil diameter that enhance light sensitivity, dim-light image formation, and spatial resolution, but sacrifice depth of focus and accommodative lens movement (Schmitz and Wainwright 2011). Similar optical traits are observed in nocturnal shorebirds (Rojas et al. 1999a, Thomas et al. 2006). In addition to temporal partitioning of resources, the variables that influence the underwater visual environments described above, such as turbidity or dissolved organic matter, can be strong drivers of the evolution of animal visual systems. Some estuarine fish species, such as the flathead grey mullet (Mugil cephalus), living in waters with high suspended-sediment loads and associated lower ambient light levels, also exhibit morphological traits (e.g., high rod density in the retina) that support scotopic (dim-light) vision. Euryhaline and diadromous fishes tend to have mixed photopigment systems that allow them to adapt to varying light environments encountered throughout their life histories (Toyama et al. 2008).
Visual Sensitivity
The sensitivity of animal visual systems to the amount and color of light in an environment depends on the type, number, spectral characteristics, and distribution of photoreceptors in the retina (Fig. 2; Lamb et al. 2007, Lamb 2013, Le Duc and Schöneberg 2016). Visual photopigments bind with photons via opsin proteins (G protein coupled receptors) bound to an inactive form of photosensitive vitamin A-based chromophores (Wald 1935). Cone opsin classes differ in wavelength sensitivity, and multiple classes of cone opsins are required for color vision. The photopigment rhodopsin evolved in a common metazoan ancestor, and photoreceptors have evolved independently along multiple invertebrate and vertebrate lineages (Yokoyama 2008). Aquatic invertebrates exhibit a remarkable diversity of photoreceptors, deriving from ciliary (e.g., polychaete tubeworms), cnidarian (e.g., cephalopods, corals), rhabdomeric (e.g., arthropods, molluscs), and Go/RGR (e.g., scallops) opsins that vary in function (Shichida and Matsuyama 2009). Molluscs, arthropods, and vertebrates possess rhabdomeric melanopsins, which support circadian rhythms, pupillary reflex, and other non-image forming functions (Shichida and Matsuyama 2009). Rhabdomeric Gq opsins allow for color vision in arthropods (Koyanagi et al. 2008). Vertebrates possess two kinds of ciliary photoreceptors: rods (Rh1) are responsible for scotopic vision, and cones (long-wavelength sensitive, LWS; short-wavelength sensitive, SWS1, SWS2; and, rhodopsin, Rh2) provide for color discrimination and visual acuity (i.e., photopic vision). Other visual opsins in vertebrates include the melanopsins and opsins of the pineal subfamily (governed by PARA, PARE, and PIN genes). Whereas humans are limited to three photoreceptor classes for color vision (e.g., red [LWS], green [Rh2], and blue [SWS]), birds and many shallow-water fishes have retained all four classes of cone visual pigments for tetrachromacy. Sabbah et al. (2013) suggest that this allows fishes to efficiently process signals in the higher spectral complexity of underwater light environments. Nocturnality in birds is associated with rod-dominated (80–90%) retinas compared to diurnal species (20–30%; Le Duc and Schöneberg 2016). A similar retinal composition is present in waterbirds, such as Adelie (Pygoscelis adeliae) and Emperor (Aptenodytes forsteri) Penguins that have adapted to extreme seasonal light cycles (Le Duc and Schöneberg 2016).
Estuarine fishes are known to have rhodopsin and porphyropsin photopigments, which vary in their spectral absorption properties. Light-sensitive photopigments are composed of opsin bound to an A1 chromophore to make rhodopsin (λmax = 500 nm) in marine fish or bound to an A2 chromophore to make porphyropsin (λmax = 525 nm) in freshwater fish (Toyama et al. 2008). Mixed photopigment systems that express A1 and A2 photopigments are common in freshwater, diadromous, and certain coastal-marine fishes that adapt to varying light environments throughout their life history. In these species, ratios of porphyropsin and rhodopsin are generally dependent on ambient light and spawning habitat (Toyama et al. 2008). Euryhaline fishes like a the gray snapper (Lutjanus griseus) and b common snook (Centropomus undecimalis) exhibit greater sensitivity toward longer or shorter wavelengths along the freshwater-marine gradient of an estuary. The changing proportion of these photoreceptors allows diadromous fishes to adapt to their environment during ontogenetic changes between marine and freshwater habitats (Allen and McFarland 1973, Robinson et al. 2011). ALAN implications: Artificial light spectra will theoretically stimulate the photopigments of freshwater, euryhaline, and marine fishes to varying degrees. Marine fishes are expected to be especially sensitive to light-emitting diodes (LEDs; see Fig. 4 for details on intensity and wavelength), which emit high-irradiance spectra that can be readily absorbed by rhodopsin. Although low-pressure sodium (LPS) lamps are commonly used to minimize ecological impacts (e.g., disorientation of sea turtle hatchlings), certain freshwater and euryhaline fishes would still perceive this narrow-spectrum lighting (Bird et al. 2004, Davies et al. 2012). Thus, LEDs might be expected to elicit behaviors typical during daylight in some fishes. For example, Becker et al. (2013) observed that large, predatory fish were more abundant on nights when a LED light was turned on, mimicking diurnal predatory activity. Whether such short-term individual behavioral responses would be favored over time would likely depend on the consistency of exposure to ALAN and the concomitant behavioral responses of their prey. The long-term, evolutionary impacts of ALAN on visual sensitivity, further enhancing the ability of some fishes to thrive under ALAN, are not known
The ability to tune photoreceptors, either by change to the molecular configuration of opsins or expression of different opsin genes, allows aquatic animals to be more sensitive to specific wavelengths of light. Spectral tuning specifically refers to plastic or evolutionary change in peak sensitivities of visual pigments in response to the photic environment (Carleton 2009). Plasticity in opsin gene expression allows for tuning of visual pigments to different light environments (Viets et al. 2016). Evolutionary shifts in mammals and birds from nocturnal toward diurnal habits led to a loss of sensitivity to UV irradiation (Hunt et al. 2009) and increased sensitivity to longer wavelengths that correspond with crepuscular light in forests (Endler 1993). These shifts were likely adaptive in preventing retinal damage from UV exposure. In contrast, the SWS1 in gulls (Laridae) are tuned to UV (Hastad et al. 2009), which may aid foraging in highly polarized bright light. Fluidity in opsin gene expression has led to a range of sensitivities across taxa, yet certain patterns have emerged for organisms that live in aquatic environments.
Blue light (~ 480 nm) plays a primary role in non-image-forming photoreception in marine and terrestrial vertebrates as the dominant light available in deeper water (λmax, 470–490 nm; Lythgoe 1979) and in terrestrial environments at crepuscular intervals (λmax, 450–500 nm; Munz and McFarland 1973; Endler 1993). Circadian rhythms (also see “Biological rhythms: circadian activity levels”, below) in aquatic organisms are thought to have coevolved with blue-light photoreception (Erren et al. 2008). For example, the blue-sensitive pigment PIN expressed in the pineal gland of birds plays a role in controlling avian biorhythms. Other cases of spectral tuning have been linked to aquatic environments. For instance, expression of the RH1 opsin has undergone spectral tuning in marine mammals as related to water turbidity (Borges et al. 2015). Visual specializations (i.e., opsin gene expression and spectral tuning) associated with habitat and temporal niches are expected to result in species-specific responses to common artificial light spectra (Fig. 3). However, blue emissions, which are included in some broad-spectrum artificial lights, may influence circadian functioning in many aquatic and terrestrial organisms.
Visual Orientation: Polarotaxis
Polarotaxis is the ability in some animals to orient based on the angle of the sun’s rays. Polarization sensitivity or adaptive camouflage in polarized habitats are understudied organismal responses that can have consequences for individual fitness and predator-prey interactions. For instance, polarized light can provide cues for spatial navigation and habitat selection important to animals sensitive to polarized light, such as aquatic and terrestrial insects (Boda et al. 2014, Perkin et al. 2014a), fishes (Hawryshyn 2010, Kamermans and Hawryshyn 2011, Pignatelli et al. 2011), birds (Muheim 2011), and bats (Greif et al. 2014). Polarization detection depends on the presence of a photopigment that is sensitive to either UV or short wavelength light (e.g., less than 400 nm). The freshwater crustacean Daphnia pulex exhibits sensitivity to polarized light although this ability is lacking in its congeners (Flamarique and Browman 2000), indicating that polarotaxis can be species-specific in aquatic macroinvertebrates. Polarization sensitivity can be especially important for aquatic insects active during crepuscular light intervals (Bernath et al. 2004) or inhabiting environments characterized by high UV (< 360 nm) such as clear, oceanic waters or yellow-green (550 nm) light (Schwind 1995), such as waters with dense phytoplankton growth. Polarized light created by reflective non-water surfaces (e.g., asphalt, glass) can create “ecological traps” for polarotactic insects (Horváth et al. 2009, Boda et al. 2014). For example, flying adult aquatic insects returning to water for oviposition detect the horizontal polarization of water-reflected light under natural conditions, but under ALAN are instead often attracted to urban light sources and horizontally polarizing non-water surfaces (Robertson et al. 2010).
Polarization sensitivity can allow predators to perceive prey as more conspicuous (i.e., higher contrast) in polarized habitats (Shashar et al. 2000). Some open-ocean fishes have modified reflective platelets that support polarocrypsis from predators (Brady et al. 2015). Polarization sensitivity has also been studied relative to habitat orientation by estuarine fishes—primarily salmonids and cyprinids. Species with specialized UV-sensitive SWS1 cone photoreceptors can detect cues primarily from celestial polarization that help in mapping natal stream location (Hawryshyn 2010). As migratory Pacific salmonids adapt from living in freshwater to saltwater (i.e., during smoltification), UV-sensitive cones undergo apoptosis to prevent retinal damage in clearer coastal environments but are later regenerated as mature adults migrate back upstream to natal habitat (Quinn 2004).
Biological Rhythms: Circadian Activity Levels
Temporal shifts in natural light (e.g., daily, seasonal) structure internal circadian rhythms that drive many physiological processes and behaviors of visual and light-detecting organisms. Diel patterns of light create optimal and suboptimal periods for activity by aquatic-associated animals. For example, the polarotactic detectability of water by aquatic insects (governed by solar elevation) during mid-morning, early afternoon, and dusk make these optimal periods for dispersal (Csabai et al. 2006). Furthermore, the duration of these optimal dispersal periods varies latitudinally. Changes to the “polarization sun dial” (sensu Csabai et al. 2006) induced by ALAN may have a stronger impact for aquatic insects in tropical systems where morning and evening periods are shorter.
Variability in light intensity throughout the lunar cycle directly influences foraging behavior by aquatic-associated organisms. During a full moon (Fig. 4), some nocturnal seabirds tend to forage for shorter intervals, whereas foraging activity is extended for some diurnal species (Tarlow et al. 2003, Navara and Nelson 2007). A breakdown of natural light cycles disrupted by ALAN may influence temporal niche partitioning, stemming from changes in foraging and predator-avoidance behaviors. Although here we focus on potential effects of ALAN on diel activity patterns, light also plays a key role in physiological and molecular pathways that influence seasonal breeding, migration, and orientation (reviewed in Dominoni 2015).
Emission spectra of solar (source: Lamp Spectral Power Distribution Database) and lunar light (source: Moon-Olino.org). From a visual perspective, ALAN increases the intensity (measured in lux or irradiance) of nocturnal light environments and shifts the spectral distribution typically to longer wavelengths (Johnsen et al. 2006, Cronin and Marshall 2011). ALAN can illuminate the nocturnal environment to intensities ranging between that of nautical twilight to brighter than a full moon on clear nights by more than 2 orders of magnitude (Kyba et al. 2011, Gaston et al. 2013, Bolton et al. 2017). Without moonlight, ALAN can exhibit peak fluxes at 560, 590, 630, and 685 nm (Johnsen et al. 2006)
The impacts of ALAN on circadian activity levels of avian consumers relate strongly to feeding strategies. Shorebirds and wading birds are resident and transient consumers in estuaries and coastal wetlands. Common taxa—such as herons, egrets, sandpipers, and plovers—vary in their visual capabilities, feeding strategies (visual or tactile), and diel activity patterns. Wading birds with weaker photopic vision capabilities (e.g., night herons and spoonbills) more often forage at night. Among the shorebirds, plovers (Pluvialis and Charadrius) and sandpipers (Scolopacidae) are cathemeral, or actively forage for periods during both day and night. Aerial insectivores such as pratincoles (Glareola) and swallows (Hirundinidae) are particularly active during crepuscular periods. Night-feeding can help meet energetic requirements and minimize encounters with diurnal predators (reviewed in McNeil and Rodríguez 1996) and while the introduction of ALAN may directly impact visually feeding shorebirds by enabling longer foraging intervals, this benefit can also extend to diurnal predators.
ALAN and the Estuarine Lightscape
The increasing intensity of ALAN, especially in coastal zones (Davies et al. 2016), is expected to disrupt natural light-dark cycles by introducing light during historically dark periods, and by providing enough light for day-active animals to see at night. The lightscape of estuarine habitats is extremely complex and thus is expected to be especially sensitive to ALAN: organismal-to-community responses to light pollution will likely vary throughout the freshwater-marine ecotone. Artificial lights can emit light in narrow or broad-spectrum bands (Fig. 3), the latter more closely mimicking natural light (Fig. 4). Davies et al. (2013) compared low- and high-pressure sodium, light-emitting diode (LED), and metal halide (MH) lamps to determine potential overlap with the visual sensitivity of arachnids, birds, insects, mammals, and reptiles to these different light spectra. Broader-spectrum emissions (e.g., high-pressure sodium, light-emitting diode, and metal halide lamps) are expected to have profound ecological effects because more species can perceive this spectral range. In particular, the increasingly popular LED lights produce broad-spectrum white light of sufficiently high intensity across the visible spectrum to allow color-mediated vision in a range of taxa from spiders to mammals (Davies and Smyth 2018). However, few comparisons of spectral perception to natural or artificial light have been drawn among riparian, freshwater, and marine taxa (Nightingale et al. 2006, Toyama et al. 2008). Building upon this approach would support theoretical understanding of physiological and behavioral responses to artificial lightscapes in estuaries. In addition, depth, turbidity, benthic substrate, and other habitat variables that define estuarine gradients may serve as useful indicators in forecasting the ecological effects of ALAN.
Consideration of the sensory environment in the context of conservation and management is an emerging field (Madliger 2012; Cooke et al. 2013; Blumstein and Berger-Tal 2015). We expect physiological and behavioral responses to the visual environment to inherently depend on the amount and spectral composition of artificial light as well as the visual sensitivity of individual organisms (Fig. 2). Growing evidence indicates that disruption to the visual environment through human-induced changes (e.g., elevated turbidity, eutrophication) leads to loss of biodiversity and alteration of communities (e.g., Seehausen et al. 1997, Seehausen et al. 2008). Our knowledge of natural nighttime light environments and communities also continues to expand (e.g., Veilleux and Cummings 2012), providing a more complete understanding of vision-mediated community dynamics; however, an understanding of how ALAN might shift those dynamics from a mechanistic (i.e., individual) level is lacking. This is particularly important for estuaries and other coastal areas, which are experiencing human population growth at a rate three times greater than the global average (Small and Nicholls 2003), with concomitant increases in ALAN.
Individual-Level Responses to ALAN
Individual-level responses to ALAN span the range of light-mediated behavioral and physiological processes. Variability in light-detection abilities (as described above) among individuals, developmental stages, populations, and species sets the stage for a wide range of responses under ALAN. After light information is detected and processed in the brain, a physiological cascade can be triggered that can disrupt hormone production (e.g., cortisol, melantonin, gonadotropins), associated biological rhythms (e.g., circadian, reproductive), and behavior (e.g., Ouyang et al. 2018, Sanders and Gaston 2018). Here, we explore examples of individual-level responses to ALAN and provide some initial examples of how behavioral shifts might help us to link responses to populations and communities (Fig. 1), a subject we address explicitly in the subsequent section (“Scaling-up from individual to ecosystem-level impacts”).
Biological Rhythms and Hormones
Biological rhythms are often informed by predictable variability in the light regime—whether through daily light-dark schedules, moon phases, or seasonal shifts in photoperiod. The physiological and behavioral processes driven by these cycles are controlled by hormonal cascades. Thus, a key individual-level response to ALAN, with direct (but as of yet untested) links to fitness and population- and community-level processes, is a change in biological rhythm. As one example, by disrupting the natural light-dark daily cycle, ALAN can act as an endocrine disruptor (reviewed in Ouyang et al. 2018). Under natural conditions, melatonin (a hormone involved with regulating daily physiological and behavioral activities) is upregulated in diurnal animals with the onset of dark, leading to decreased activity, but under ALAN, melatonin production may be inhibited by the artificial light signal (Ouyang et al. 2018). This can have profound effects on timing of activities, which are typically adapted to be optimal at particular times of the day or night (e.g., when food is most available), potentially resulting in reduced fitness. In Atlantic salmon (Salmo salar), for example, fry exposed to a moderate level of ALAN meant to mimic street lights (12 lx) were smaller and dispersed later than fry held under normal light/dark cycles (Riley et al. 2013). Additionally, the timing of fry dispersal from emergence to feeding territories was compromised; instead of dispersing at night, ALAN-treated fry dispersed throughout the day, an activity that could lead to reduced survival via increased predation or inferior feeding territories. Even at very low levels of ALAN (~ 1 lx), Eurasian perch (Perca fluviatilis) showed inhibited melatonin production and higher nighttime activity (Brüning et al. 2015). Similarly, a passerine bird, the great tit (Parus major), experimentally showed depressed melatonin levels and associated increased nighttime activity when exposed to ALAN (de Jong et al. 2016). In these examples, the intensity of light was the important signal leading to decreased melatonin and a change in timing of activity; however, further work has shown that the spectral content (or color) of light can have differential effects on endocrine function (Brüning et al. 2016). Eurasian perch exposed to different colors of light showed differentially reduced melatonin production, with blue (short wavelength) light inhibiting production the least (though all colors and intensities showed reduced melatonin relative to the dark control). Interestingly, Brüning et al. (2016) also found sex-specific differences in gonadotropin (reproductive hormones) production under white light: exposed to only 1 lx of white (broad spectrum) light, female perch showed suppressed gonadotropin gene expression. The implications for this could include reduced fecundity and fitness.
Stress response is another endocrine pathway of potential concern for ALAN-affected species, which is facilitated by elevated glucocorticoid hormones (e.g., cortisol). Long-term exposure to stress with elevated levels of glucocorticoids can lead to compromised immune responses, regulation of metabolism, and decreased reproductive output. In one of the few studies assessing stress responses to ALAN, Migaud et al. (2007) found that Atlantic salmon exposed to high intensities of blue LED light showed elevated cortisol within 3 h of exposure. Cortisol levels then dropped after 24 h of exposure, suggesting an acute response. While studies of glucocorticoid expression and the potential impact for individuals (with potential links to population-level changes) experiencing environmental stressors are increasing (see Dantzer et al. 2014), the results for the few studies studying stress responses to ALAN are somewhat equivocal. For example, Brüning et al. (2015) found no effect of white ALAN on cortisol levels in Eurasian perch, while Ouyang et al. (2015) found elevated corticosterone in songbirds exposed to similar levels of white street lighting. More studies that test the effects of multiple colors and intensities of ALAN across a variety of taxa, especially across estuarine gradients, would be informative in the pursuit of an underlying mechanism that could inform predictions about population responses.
Behavioral Responses
One of the classic cases demonstrating the detrimental impacts of ALAN on animals was the observation that newly hatched sea turtles orient and move toward artificial lights rather than toward moonlight and starlight reflecting off the water’s surface (Butler 1998). This behavior was implicated in the decline in sea turtle populations, likely through both failure to reach the sea and enhanced predation pressure. There is now strong evidence that light-mediated behaviors across a diverse array of taxa will be influenced by ALAN (e.g., habitat choice and use, migration, foraging, competition, reproductive behavior, predator-prey interactions; reviewed in Gaston et al. 2015, Davies and Smyth 2018). We can draw from a number of examples in both aquatic and terrestrial animals to illustrate that behavioral shifts might be an extremely important link between individual responses, interspecific interactions, community dynamics, and ecosystem functioning (Fig. 1).
Reproductive cycles are directly tied to endocrine pathways, and because these pathways are disrupted by ALAN, we also expect to observe shifts in reproductive behavior. Songbirds, for example, show enhanced gonadal development (Ouyang et al. 2018), sing earlier in the morning and receive higher extra-pair copulations when near street lamps (Kempenaers et al. 2010). In freshwater, male nest guarding in smallmouth bass (Micropterus dolomieu) was also impacted by ALAN: nesting males exposed to ALAN (either continuous low intensity dock lights or intermittent high intensity light mimicking car headlights) displayed more intense guarding behavior than males not exposed to ALAN (Foster et al. 2016). The energetic repercussions of increased guarding activity could lead to reduced reproductive success and population-level consequences. Altered underwater light environments are known to contribute directly to the loss of biodiversity in aquatic systems, for example via disruption of visual signals used for mate choice during reproduction (van der Sluijs et al. 2011). In these cases, light intensity was decreased, and color content shifted due to elevated turbidity levels; however, since ALAN provides more light at night, and in particular broad-spectrum light that allows for color-mediated behaviors, it is unclear if reproductive output will always be compromised directly.
The impact of ALAN on migration can be both direct and indirect. Direct effects include changing the timing and path of migration. For example, unmanaged exposure to ALAN along precarious migratory routes through estuaries (Stich et al. 2015) may potentially impede navigation that leads to successful recruitment of salmonids to natal upstream habitats (Mueller and Simmons 2008). Diel migration of zooplankton (e.g., copepods) can similarly be directly affected by ALAN through an inhibited upward migration at night (reviewed in Davies and Smyth 2018). Indirect effects on migratory behavior stem from the potential change in predator behavior along ALAN-infiltrated migration routes (reviewed in Nightingale et al. 2006). For example, juvenile salmon were more heavily preyed upon by sculpin (Cottus spp.) during outward migration in areas of intense ALAN (Nightingale et al. 2006). It is apparent from these examples that behavioral shifts can be linked to population-to-community level responses.
Changes to intra- and inter-specific behavioral interactions are expected to be especially important in determining how individual responses to ALAN scale to changes observed in community structure and function. For example, predator-prey relationships contribute to the structuring of communities, thus any change in the behavior of either prey or predators may have profound effects on an ecosystem. As an example, in certain migratory bat species (Pipistrellus spp.), different colored lights elicited different behavioral responses: red LED light resulted in increased activity but not foraging behavior, while exposure to white LED light produced the opposite result, with increased foraging behavior and no change in activity (Voigt et al. 2018). Coupled with evidence of shifts in the abundance of insect emergence from streams with ALAN (Meyer and Sullivan 2013), such impacts on bat foraging behavior could further alter observed community dynamics in stream-riparian subsidies. By augmenting competition among visual diurnal competitors and predators that often feed under ALAN, nocturnal species are likely to experience changes in foraging effort and energetic intake. In estuary tidal zones, the influence of ALAN will interact with the way individuals contend with nighttime tides. For instance, foraging activity of tactile-feeding shorebirds (e.g., Scolopacidae) closely follows the tides, which determine foraging habitat and prey availability (Le Duc and Schöneberg 2016). However, under ALAN, the distribution of prey, competitors, and predators shifts. Estuaries represent critical habitat for threatened migratory shorebirds that must maximize their energetic intake during narrow stopover intervals in order to successfully travel long distances between breeding and wintering habitats. In an estuary of Northern Europe, ALAN influences stopover habitat selection and foraging behavior of the common redshank (Tringa totanus), with more opportunities for nighttime visual foraging (Dwyer et al. 2013). Regions in the northeastern U.S. with high levels of ALAN attract a higher density of nocturnal autumnal migrants, which may lead to increased levels of competition for habitat and food resources during these critical periods (McLaren et al. 2018).
As the examples described above demonstrate, ALAN can interact with sensitivity to light, biological rhythms, and endocrine systems to drive individual behavior. In addition to altering nighttime behavioral patterns, daytime behaviors of aquatic organisms can also be affected by ALAN. For instance, Kurvers et al. (2018) showed that ALAN affected diurnal risk-taking behaviors in Trinidadian Guppies (Poecilia reticulate), thereby illustrating how ALAN can have consequences that extend beyond the immediate nighttime responses of animals to changes in lighting conditions. The cumulative effect of these responses should translate from the individual-level to population consequences, and to processes within communities and ecosystems.
Scaling-Up from Individual to Ecosystem-Level Impacts
Functionally linking individual-level characteristics to communities and ecosystem processes is among the biggest challenges to understanding the mechanisms of and responses to human-induced environmental change (Levin 1992, Gilbert et al. 2015, Cooke et al. 2017). ALAN has the potential to influence evolutionary and population trajectories by altering behaviors that are mediated by visual sensitivity, habitat selection and orientation, and circadian activity rhythms of aquatic organisms. Furthermore, because natural light drives primary production and trophic interactions (e.g., grazing, predation), ALAN may alter estuarine communities, with ramifications for food-web structure and ecosystem functioning (Table 1, Fig. 1). For instance, Bolton et al. (2017) observed increased predatory fish behavior under ALAN in an Australian harbor, which in turn was associated with shifts in prey fish and sessile invertebrate community structure.
Here, we consider how individual- and population-level responses to ALAN can mediate potential impacts on estuarine community dynamics and ecosystem functioning. Specifically, we address how ALAN may affect (i) individual and species-to-population and -community responses and (ii) community-to-ecosystem responses.
Linking Individual and Species Responses to Population and Community Dynamics
Individuals are expected to respond to ALAN-related changes in the light environment; thus, we expect modification of individual activities to translate into population- and community-level effects. As examples, we focus on two individual-level mechanisms—trophic-niche partitioning and movement of estuarine organisms—that are closely tied to lighting regimes and that might be expected to affect community structure under ALAN.
Temporal-Niche Partitioning
Natural light cycles structure temporal-niche partitioning in ecological communities, as community interactions are driven by energetic and risk trade-offs at different times of day. The temporal predictability of resource distribution and predation risk has led to ecomorphological adaptations for diurnal, nocturnal, or cathemeral activity. Diurnal disposition has led to the evolution of greater morphological, optical, and trophic diversity. Yet nearly 15% of all described fishes feed, spawn, or migrate nocturnally during at least one life-history stage (Hölker et al. 2010b). Although ocular adaptations allow for optimal visual performance at certain diel intervals, the strength of competitive interactions also drives optimal foraging strategies (Brown et al. 1999). The constriction or expansion of diel niches can largely depend on shifts in predator communities (McCauley et al. 2012) and may contribute to the avoidance of competitive exclusion and mediate coexistence among predators, prey, and competitor species (Kronfeld-Schor and Dayan 2003). Diel-niche partitioning based on differences in photopic vision capabilities has been suggested as a mechanism that reduces competition among diurnal (e.g., great egret, Ardea alba), cathemeral (e.g., roseate spoonbill, Platalea ajaja), and nocturnal (e.g., black-crowned night heron, Nycticorax nycticorax) wading birds (Britto and Bugoni 2015). Under ALAN conditions, we may then expect a reorganization or a breakdown of temporal niche partitioning with implications across the estuarine community.
ALAN also has the potential to alter competitive interactions via changes in day-night activity of predators, prey, and competitors in estuarine communities (Fig. 5). For example, ALAN disrupts orientation of sea -turtle hatchlings, yet it may also increase hatchling predation by birds, reptiles, and mammals as the hatchling turtles disperse from nest to sea. Sea-turtle nesting beaches are among the few, if not the only, nearshore aquatic habitats managed for ALAN intensity and spectra (Butler 1998). However, early studies on light pollution gleaned some understanding of its effects on community interactions on coastal beach habitats; researchers observed limited foraging (i.e., number of seeds harvested) and reduced patch preference for ALAN-treated (incandescent and LPS lighting) habitat by the nocturnal beach mouse (Peromyscus polionotus leucocephalus) (Bird et al. 2004). Later studies attributed this response to a heightened perceived risk of predation (Falcy and Danielson 2013, Wilkinson et al. 2013). By influencing risk trade-offs for individuals, ALAN may transform intraguild competitive interactions. Diurnal mice congeners in this habitat were not more active under ALAN conditions (Rotics et al. 2011), implying that both species may have faced increased daytime competition for resources. In some scenarios, using ALAN to induce these behavioral shifts may provide a unique management approach. For example, exposing the nocturnal signal crayfish (Pacifastacus leniusculus) to high-pressure sodium (HPS) lighting at night inhibited activity and competitive interactions with native crayfish species (Thomas et al. 2016). However, chronic exposure to ALAN may lead to habituation and thresholds (intensity or duration) at which behaviors depend on physiological sensitivity and predation-risk regime. Facilitative diurnal foraging by nocturnal fishes has been observed in predator-depauperate reefs in the Pacific atolls (McCauley et al. 2012) suggesting that, in certain cases, predation risk can be a stronger force in structuring day-night communities than visual sensitivity. Facilitative nocturnal foraging by diurnal predators has been observed with wading birds (Santos et al. 2010, Dwyer et al. 2013) and fishes (Becker et al. 2013) in estuarine habitats with implications on prey activity. Understanding the short-term and long-term effects of ALAN on competitive interactions will be valuable in predicting how communities will respond to this environmental stressor over time.
Cross-boundary fluxes of prey represent a key mechanism linking terrestrial and aquatic ecosystems (reviewed in Baxter et al. 2005, Sullivan and Rodewald 2012). Here, we highlight the potential influences of ALAN on avian consumers in estuaries that feed on both aquatic insects (larval and emergent), other aquatic invertebrates, and fish. In particular, wading birds (i.e., shorebirds and long-legged waders) are both permanent and transient residents in temperate and tropical estuaries. Waders are highly effective visual and tactile foragers (with color vision mediated by four cone photoreceptor classes) and often exhibit sensitivity to UV light (Hart 2001a). There are important differences in the visual morphology of wading birds associated with foraging tactic and time of day (McNeil et al. 1992, Thomas et al. 2006). Visual foragers that feed both during the day and night (e.g., plovers and stilts) have a higher density of retinal photoreceptors compared to tactile-feeding sandpipers (Rojas de Azuaje et al. 1993, Rojas et al. 1999a). More specifically, species that forage at crespuscular or nocturnal periods have greater rod densities and rod/cone ratios (Rojas de Azuaje et al. 1993, Rojas et al. 1999a, b, McNeil et al. 2004). A visual system most sensitive to wavelengths in which up-welling light from the water is rich and surface reflectance relatively poor (425 to 500 nm for clear blue oceanic water) is best suited for seeing through the water surface (Lythgoe 1968), but that is rarely observed. Birds that look through an aquatic surface to locate prey tend to have a relatively high proportion of long-wavelength-sensitive cones and yellow-red ocular filters (Hart 2001a, b). ALAN implications: Collisions with lighted structures over land (e.g., buildings with reflective surfaces) and at sea (e.g., vessels) represent the most direct impact of ALAN on aquatic birds. These events are especially common in urban areas, posing an additional threat to nocturnal migrants including shorebirds and wading birds that tend to fly at lower elevations (Evans Ogden 1996, Loss et al. 2014, Rodriguez et al. 2017). Indirect effects of ALAN on aquatic-associated birds are less understood; however, understanding their visual physiology may help glean insight on potential responses. Analysis of spectral sensitivity of 16 avian species to artificial light spectra suggests that, like other visual organisms, they would be most affected by LED lighting and less by LPS and other lights with long-wavelength shifted spectra (Davies et al. 2013). This analysis was largely based on songbirds and future research should address whether visual sensitivities of aquatic-associated birds may lead to similar or distinct response. One of the most likely responses of wading birds may be an increase in activity throughout the night under ALAN (Gaston et al. 2013), which may exert additional top-down pressures on fish and aquatic invertebrates with concomitant repercussions to aquatic ecosystems, such as estuaries (e.g., predation-induced reductions in densities of herbivorous fish and invertebrates may release aquatic primary producers from grazing pressure and lead to an increase in primary productivity)
Natural lighting regimes have been shown to drive activity and distribution of different size and trophic classes in estuarine fish communities. In estuarine mangroves, for instance, nocturnal fish assemblages are often composed of planktivores and piscivores, whereas detritivores have been shown to dominate diurnal communities (Ley and Halliday 2007). In an estuary of South Africa, Becker et al. (2013) used acoustical survey methods to record the effect of a sodium-vapor floodlight on the abundance and behavior of different size classes in the fish community. Observations included a shift from exploratory behavior or foraging activity to more vigilant behavior by smaller fishes when exposed to nocturnal lighting, while larger predators took advantage of a sit-and-wait foraging tactic at the edge of the light-dark boundary. A change in species-specific predatory behavior altered community dynamics by reorganizing the size structure of the nocturnal fish assemblage.
Both terrestrial and aquatic species that rely heavily on prey with phototaxic behaviors (e.g., aquatic insects) often demonstrate facultative nocturnal activity to exploit ALAN-related foraging opportunities. An increase in predator-prey interactions mediated by ALAN could potentially redefine optimal-foraging strategies. Because many swimming prey and invertebrates in muddy estuarine habitats are closer to water and sediment surfaces at dusk and at night, visual predators may forage more efficiently in estuarine habitats under artificial lightscapes. Visually feeding wading birds that forage opportunistically under artificial light are thought to exert low predation pressure (i.e., lower catch success) compared to daytime foraging (Santos et al. 2010, Dwyer et al. 2013), yet nocturnal activity may have non-consumptive indirect effects. Yeager et al. (2016) observed that the simulated presence of a wading bird tripled foraging interactions between mangrove crab (Aratus pisonii) and a euryhaline mesopredator, the gray snapper (Lutjanus griseus; λmax = 513 and 560 nm, McComb et al. 2013). As crabs move to lower-root habitat structure to avoid detection from wading birds, they may become prey for visual fish predators. The strength of this and similar interactions should vary depending on the spatial and temporal distribution of prey and predators (Alvarez et al. 2013) throughout an estuary with implications for top-down trophic effects.
Dispersal, Migration, and Foraging Movement of Estuarine Organisms
Dispersal of riverine and marine invertebrates is a vital mechanism driving population dynamics and habitat connectivity in estuaries (Chew et al. 2015). By affecting aerial dispersal of adult aquatic insects (Horváth et al. 2009, Boda et al. 2014), ALAN hinders insect recruitment and prey availability for higher consumers (Horváth et al. 2009, Robertson et al. 2010). The potential consequences of ALAN on aquatic dispersal dynamics (i.e., organisms moving/dispersing through the water) are less clear. Dispersal of planktonic invertebrates and larvae are passive processes driven by the physical movement of water (i.e., downstream, tidal, and surface current flow; reviewed in Norcross and Shaw 1984). In estuaries, these processes are mediated by tidal dynamics (i.e., timing, stratification, and mixing). Passive dispersal and active migrations by zooplankton and mobile consumers are also strongly linked with seasonal and diel light cycles (Brittain and Eikeland 1988, Palmer et al. 1996). For example, larval invertebrate drift in freshwater systems has been shown to peak during nocturnal periods, which aligns with lower predation risk (Flecker 1992, Miyasaka and Nakano 2001, Hernandez and Peckarsky 2014). If predator activity increases under ALAN, nocturnal drift communities may face elevated perceived risk of predation. Although nocturnal activity generally persists even if predators are experimentally or naturally excluded (Flecker 1992, Hampton and Duggan 2003), foraging and drift behaviors may vary based on the type of predator (e.g., active visual predators vs. smaller tactile predators; Peckarsky and McIntosh 1998). Nocturnal drift activity by stream invertebrates reduced following the addition of ALAN (Henn et al. 2014, Manfrin 2017), suggesting that ALAN can enhance, or exert a stronger effect than, predation risk on nighttime foraging and dispersal.
Zooplankton exhibit passive and active migration behavior that varies across tidal, diel, and lunar cycles. For example, in tropical estuaries, diel vertical migrations are induced by light and tidal patterns; these dynamics can differ among species based on salinity tolerance (Chew et al. 2015). Species adapted to lower salinity conditions ascend for nocturnal flood tides and descend for diurnal ebb tides whereas euryhaline and stenohaline species exhibit the opposite behavior to maintain optimal water-column position in the estuary. In a North Wales estuary, macroinvertebrates in the drift in a tidal freshwater area were also spatially distributed based on salinity tolerances. Freshwater chironomids, caddisflies, stoneflies, and mayflies drifted downstream whereas marine copepods and oligochaetes often exhibited “reverse” drift as the flood tide moved them upstream (Williams and Williams 1998). If the onset of drift behaviors is interrupted due to ALAN-mediated changes in predator behavior, estuarine invertebrates could experience physiological stressors (e.g., osmotic stress) that influence fitness. Light is often the primary factor regulating migrations for nocturnal and euryhaline species (Flecker 1992). Notably, the synchronization of vertical positioning within the water column and tidal regime determines upstream-downstream movement and retention within the estuary (Chew et al. 2015). Even minor changes in light can influence migration patterns (Haney 1993, Ringelberg 1999). Thus, spill-over of ALAN into estuarine habitats may desynchronize this process with consequences for recruitment, community composition, and adjacent trophic levels. For example, if zooplankton are largely confined by ALAN to deeper depths than under natural lighting conditions, prey availability to surface planktivores (e.g., nocturnally adapted juvenile and other planktivorous fishes) is likely to be reduced.
Few studies have addressed how an ALAN-shifted nocturnal plankton community may affect higher trophic-level consumers. Artificial light-addition treatments have demonstrated potential shifts in the abundance and community structure of drift invertebrates (Henn et al. 2014). In freshwater streams, experimental light treatments (HPS) reduced the density of night-time drift by about 50% but did not affect drift-foraging cutthroat trout (Oncorhynchus clarkii) growth rates (Perkin et al. 2014b). Despite impacts on invertebrate prey during this short-term study, predator responses may take longer to detect than those of primary consumers. In the short-term, ALAN can extend foraging conditions for visual predators, allowing them to compensate with alternative resources.
Natural light also regulates dispersal, diel migrations, and circadian behaviors of many coastal and estuarine fishes throughout ontogeny (Bradbury et al. 2006, Naylor 2006, Epifanio and Cohen 2016). For example, nocturnal movements by adult and juvenile grunts (Haemulidae) to foraging habitats are synchronized by size class and cued by changes in underwater light level (McFarland et al. 1979). Similarly, juvenile sockeye salmon (Onchorhyncus nerka) exhibit diel vertical migration to maintain position in an optimal light environment that minimizes their exposure to predation (Scheuerell and Schindler 2003). Artificial light has the potential to mask circadian light cues and thus disrupt the adaptive significance of these behaviors (Kurvers and Hölker 2015). For example, elevated ALAN intensities in mesocosm trials led to a delay and desynchronization of fry dispersal of Atlantic salmon (Riley et al. 2012, 2015). In natural systems, a disturbance of temporal movement patterns of fry would have implications for larval and juvenile survivorship due to increased risk of predation (Stich et al. 2015).
Seasonal and diel migrations by consumers can also affect estuarine connectivity (Nagelkerken 2009, Rosenblatt et al. 2013, Sheaves et al. 2015). Although there is inter- and intraspecific variability in diel foraging among estuarine fishes (Ramirez-Martinez et al. 2016), many species (e.g., Lutjanidae) seek refuge in tidal mangroves during the day and migrate at night to feed in soft-bottom habitats. As another example, nocturnal foraging by reef-dwelling grunts (Haemulidae) is temporally partitioned; migration from resting to foraging habitats occurs chronologically for groups at different stages of ocular development (McFarland et al. 1979, Robinson et al. 2011). The spectral composition of light changes rapidly during dusk, requiring the eye to adjust to a blue-green dominated environment. During dusk, retinomotor movement (specifically the shift from stimulation of yellow-orange cones to predominantly blue-green cones in the retina) affects the timing and duration of foraging migrations by grunts traveling among coastal reef, mangrove, and seagrass habitats (McFarland et al. 1979, McFarland and Wahl 1996). Disruption or delay of visual adjustment under ALAN conditions may inhibit foraging activity and increase predation risk at diel or ontogenetic intervals of poor visual acuity. Studies have highlighted the stark changes in fish communities that occur across a day-night period (Zapata et al. 2014), as well as their importance for nursery functioning (Nagelkerken et al. 2000), suggesting that dark periods can support species coexistence and high biodiversity. The introduction of ALAN is likely to lead to the restructuring or loss of diel community turnover.
Linking Community Responses to Ecosystem Processes: Food Webs as a Case Study
ALAN impacts on individuals-to-communities will play out at the ecosystem scale via its alteration of food webs and energy flow. Flows of energy represent an ecosystem process by which nutrients, organic matter, and prey are transferred across and within ecosystem boundaries (e.g., aquatic-terrestrial) by abiotic (e.g., fluvial and tidal flow) and biotic (e.g., movement of consumers) vectors. Trophic networks (i.e., food webs) reflect energy pathways, species interactions, biodiversity, and ecosystem productivity (Link et al. 2005); and thus integrate individual, species, and community responses to environmental change (Thompson et al. 2012).
Quantitative measures of food-web structure—such as food -chain length (FCL), interaction strengths, and connectance—describe the topology of trophic networks and functional ecosystem properties (Thompson et al. 2012). For example, the number of transfers of energy from basal organisms to apex predator (i.e., FCL) can interact with biodiversity to affect secondary production and biomass accumulation. For instance, in the seagrass communities of the York River estuary, predators regulate the grazer community and thus promote algal production (Duffy et al. 2005). ALAN-induced changes in biodiversity and trophic networks could contribute to declines of ecosystem functions (Hooper et al. 2012) and destabilization of coastal ecosystems (Saint-Béat et al. 2015).
In estuaries, we expect increasing levels of ALAN to alter how energy is transferred across multiple gradients (e.g., freshwater-to-marine, aquatic-to-terrestrial, and vice versa). For example, ALAN may delay leaf fall for deciduous trees (Bennie et al. 2016), affecting the magnitude and timing of nutrient inputs in the form of leaf detritus into aquatic habitats and thus shifting aquatic ecosystem metabolism. Furthermore, the relative openness of the canopy during this transition has implications for phytoplankton production (via light intensity). Increased or continuous light exposure may induce photo-saturation and -inhibition in aquatic primary producers (Henley 1993), influencing algal communities and production. For example, experimental ALAN-addition led to a 43 to 57% (seasonally dependent) decrease in overall periphyton biomass and altered community composition in an alpine stream (Grubisic et al. 2017). Thus, because exposure to ALAN and in particular to LED white lights can affect primary producer biomass and community composition in freshwater systems (Grubisic 2018), bottom-up food-web effects might be expected in estuaries as well.
Phytoplankton productivity typically varies along estuarine gradients in response to light limitation often associated with turbidity (Harding et al. 1986, Cloern 1987); as such, ALAN may induce differential impacts on carbon assimilation by phytoplankton across an estuary. Beyond its potential indirect effects on consumers via primary production, ALAN exposes terrestrial and aquatic consumers to markedly distinct spectral and temporal patterns of light, and directly influences consumer interactions that mediate aquatic-terrestrial trophic linkages. Further, ALAN is expected to advantage taxa that utilize light as a resource to locate prey, but hamper those using darkness as a resource to hide from prey or predators (Davies et al. 2012, Gaston et al. 2013). In this way, such taxon-specific responses may elicit top-down trophic impacts on community structure (reviewed in Bennie et al. 2015a).
Aquatic-Terrestrial Trophic Linkages
Food-web interactions across the aquatic-terrestrial boundary represent a high level of system integration that reflects multiple structural and functional properties of both ecosystems (Fig. 5). In freshwater systems, emergent aquatic insects have been shown to be a critical nutritional subsidy for a suite of riparian consumers including arthropods, birds, mammals, and reptiles (Baxter et al. 2005, Paetzold et al. 2005, Fukui et al. 2006). For example, emerging aquatic estuarine insects have been linked to nearshore orb-weaving spider distribution in a Florida estuary (Zapata and Sullivan 2018). Riparian birds also can be highly dependent on emerging insects, either directly as a food source or indirectly through other prey that feed on emerging insects (e.g., spiders; Alberts et al. 2013, Kautza and Sullivan 2016). Thus, ALAN impacts on organismal behavior and physiology, or on key ecosystem processes such as aquatic (or terrestrial) primary productivity and nutrient cycling is expected to propagate through linked aquatic-terrestrial food webs with cascading implications (Grubisic et al. 2017, Manfrin 2017). For instance, night lighting has been shown to influence the feeding activity of riparian bats due to increased density of insect prey attracted to light (Kuijper et al. 2008) and the foraging activity of wading birds via increased visibility of prey (Dwyer et al. 2013). Working in a stream system, Meyer and Sullivan (2013) found that higher ALAN levels were associated with an increase in the density, diversity, and body size of terrestrial arthropods entering the stream, with concomitant reductions in emergent insect body size and community diversity. Furthermore, their work suggested shifts in the timing of aquatic insect emergence, with peaks occurring later in the summer under higher ALAN levels. ALAN-induced changes in the composition, timing, and phenology of emergent insect communities in estuaries are likely to affect a suite of insectivorous species (lizards, birds, bats, etc.) and alter food-web dynamics through multiple mechanisms. For example, ALAN may affect FCL—and thus energy flow through ecosystems, nutrient cycling, freshwater-atmospheric carbon exchange, and bioaccumulation of contaminants in humans via consumption of top predators (Sabo et al. 2010 and references therein). In fact, Sullivan et al. (in press) found that invertebrate FCL was lower under ALAN in an urban stream-riparian ecosystem, in part implicating a loss of functional diversity at the community level. To a certain extent, shifts in FCL (which may be positive in some circumstances; see Sullivan et al. in press) under ALAN likely occur via greater predation by improving vision of predators, increasing abundance of positively phototactic prey, or attracting new predators (Becker et al. 2013, Bolton et al. 2017).
Management and Conservation Implications of ALAN Across Levels of Biological Organization
More than a decade after Longcore and Rich’s (2004) seminal review of ecological consequences of ALAN, governing policies that regulate artificial light emissions are rare. The well-known example of replacing broad-spectrum light with longer-wavelength light to decrease sea -turtle hatchling mortality (Witherington and Martin 2003) addressed population-level issues by understanding individual-level responses (Madliger 2012). The Dark Sky Initiative (International Dark Sky Association 2003) is now setting the stage for management of ALAN in many protected areas in the United States with the designation of “Dark Sky Parks.” The Model Lighting Ordinance (MLO) and The Illuminating Engineering Society of North America developed the Backlight-Uplight-Glare (BUG) rating system of outdoor luminaires that evaluate their performance in terms of light trespass, sky glow, and high-angle brightness (California Lighting Technology Center 2014). Together with maximum allowable BUG ratings for various lighting zones (e.g., protected areas are classified as Lighting Zone 0) developed by the International Dark Sky Association and MLO, this creates best-practice guidelines to assist managers in ensuring the appropriate lighting characteristics. The designation of Dark Sky Parks in estuaries and coastal-marine areas (Davies et al. 2016) will further encourage ALAN management practices that minimize individual-to-ecosystem-level impacts. A recent global risk assessment of light pollution impacts reported that 16.8% of protected lands, including mangrove forests, in the United States are subjected to ALAN (Aubrecht et al. 2010b). Meanwhile, an understanding of ALAN effects on the physiology and behavior of animals (Navara & Nelson 2007; Hölker et al. 2010b), community structure, and ecosystem functioning is needed to shape management priorities and strategies. Certainly, the trends of intensifying artificial lightscapes in coastal regions demand future research that quantifies ALAN effects on individual organisms and importantly, how individual responses scale-up to community and ecosystem responses.
Increasingly, research is pointing to ways to mitigate the effects of ALAN (Azam et al. 2015, Verovnik et al. 2015). Gaston et al. (2012) offer five main categories of mitigation measures: maintaining and creating dark areas, reducing light trespass, dimming, part night-lighting, and changing spectra. Restricting use of ALAN adjacent to natural areas to certain timeframes and to limited spectral ranges, for instance, allows communities to ensure public safety while minimizing ecological impacts. Part-night lighting approaches are effective in minimizing impacts on crepuscular periods, which have been shown to be important times of activity (Day et al. 2015). Whereas these strategies focus on mitigating effects on animals directly impacted by ALAN, maintaining circadian behaviors and community interactions during critical periods could support ecosystem functioning (e.g., consumer-mediated connectivity). When lighting is needed to enhance human safety, implementing technologies such as motion-sensing lights, LED lights with spectra that reduce overlap with common visual sensitivities (short wavelengths), and limited-angle lighting that reduces exposure to a narrow area could mitigate ecological impacts (Schroer and Hölker 2017). As with other ecosystem types, mitigation strategies will need to be considered in the context of both the relative weight of scientific evidence in their favor and the potential negative consequences to human communities, perceived or real.
Conclusions and Future Directions
Based on our review and synthesis of documented ALAN impacts from individuals to communities and ecosystems, we conclude by proposing the following key research avenues, which we offer as the basis for development of a cohesive research framework (also see Fig. 1):
-
1.
Characterizing natural diel and seasonal light changes in estuarine habitats (sensu Veilleux and Cummings 2012) will help predict ALAN impacts and inform management of artificial light use (intensity, spectral composition, and timing).
-
2.
Biological responses may vary with the magnitude, duration, frequency, and predictability of exposure to ALAN. Future research should work to understand these variables across aquatic and terrestrial systems with long-term controlled studies in aquatic, terrestrial, and coupled systems.
-
3.
Future research should draw connections among known effects of natural and artificial light on animal physiology and community ecology to evaluate potential effects on estuarine processes and functional responses such as the multiple dimensions of estuarine connectivity and productivity.
-
4.
Mechanistically linking ALAN-responses across levels of biological organization will be critical to developing a predictive understanding of the effects and consequences of ecological light pollution in coastal areas and beyond, and in developing effective mitigation strategies.
ALAN is an environmental stressor prevalent throughout urban and developing coastal areas. Marine protected areas (MPAs) of the Gulf of Mexico and Caribbean, Atlantic Mediterranean, eastern coast of South America, and Australia are subject to extensive ALAN (Davies et al. 2016). Trends of increasing light intensity have been linked with coastal development (Davies et al. 2014) and thus ecologically and economically important estuarine habitats will receive more light pollution in the coming decades. Yet there are opportunities to implement policy and technologies that minimize ecological impacts (Gaston et al. 2012). To better manage for joint stressors that affect estuaries (e.g., rising sea level, changing salinity regime, freshwater inputs), we must also anticipate changes to ecosystem functioning associated with ALAN. Despite the presence of ALAN in MPAs since the early 1990s (Davies et al. 2016), research is only beginning to hone understanding of the scale of this environmental stressor and its consequences on ecological processes in coastal ecosystems.
References
Alberts, J.M., S.M.P. Sullivan, and A. Kautza. 2013. Riparian swallows as integrators of landscape change in a multiuse river system: Implications for aquatic-to-terrestrial transfers of contaminants. Science of the Total Environment 463-464: 42–50.
Allen, D.M., and W.N. McFarland. 1973. Effect of temperature on rhodopsin/porphyropsin ratios in a fish. Vision Research 13 (7): 1303–1309.
Alvarez, M.F., D.I. Montemayor, M.C. Bazterrica, M. Addino, E. Fanjul, O. Iribarne, and F. Botto. 2013. Interaction strength varies in relation to tidal gradient and spatial heterogeneity in an intertidal Southwest Atlantic estuarine food web. Journal of Experimental Marine Biology and Ecology 449: 154–164.
Aubrecht, C., C.D. Elvidge, D. Ziskin, P. Rodrigues, and A. Gil. 2010a. Observing stress of artificial night lighting on marine ecosystems—a remote sensing application study. In ISPRS TC VII Symposium, 41–46. Vienna, Austria: IAPRS.
Aubrecht, C., M. Jaiteh, and A. de Sherbinin. 2010b. Global assessment of light pollution impact on protected areas. CIESIN: AIT Working Paper. Palisades, NY, USA: CIESIN and NASA SEDAC, The Earth Institute at Columbia University.
Azam, C., C. Kerbiriou, A. Vernet, J.F. Julien, Y. Bas, L. Plichard, J. Maratrat, and I. Le Viol. 2015. Is part-night lighting an effective measure to limit the impacts of artificial lighting on bats? Global Change Biology 21 (12): 4333–4341.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.W. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81 (2): 169–193.
Baxter, C.V., K.D. Fausch, and W. Carl Saunders. 2005. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology 50 (2): 201–220.
Becker, A., and I.M. Suthers. 2014. Predator driven diel variation in abundance and behaviour of fish in deep and shallow habitats of an estuary. Estuarine, Coastal and Shelf Science 144: 82–88.
Becker, A., A.K. Whitfield, P.D. Cowley, J. Jarnegren, and T.F. Naesje. 2013. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. Journal of Applied Ecology 50 (1): 43–50.
Bennie, J., T.W. Davies, D. Cruse, R. Inger, and K.J. Gaston. 2015a. Cascading effects of artificial light at night: Resource-mediated control of herbivores in a grassland ecosystem. Philosophical Transactions of the Royal Society, B: Biological Sciences 370 (1667): 20140131.
Bennie, J., J.P. Duffy, T.W. Davies, M.E. Correa-Cano, and K.J. Gaston. 2015b. Global trends in exposure to light pollution in natural terrestrial ecosystems. Remote Sensing 7 (3): 2715–2730.
Bennie, J., T.W. Davies, D. Cruse, and K.J. Gaston. 2016. Ecological effects of artificial light at night on wild plants. Journal of Ecology 104 (3): 611–620.
Bernath, B., J. Gal, and G. Horvath. 2004. Why is it worth flying at dusk for aquatic insects? Polarotactic water detection is easiest at low solar elevations. Journal of Experimental Biology 207 (5): 755–765.
Bird, B.L., L.C. Branch, and D.L. Miller. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conservation Biology 18 (5): 1435–1439.
Blumstein, D.T., and O. Berger-Tal. 2015. Understanding sensory mechanisms to develop effective conservation and management tools. Current Opinion in Behavioral Sciences 6: 13–18.
Boda, P., G. Horvath, G. Kriska, M. Blaho, and Z. Csabai. 2014. Phototaxis and polarotaxis hand in hand: Night dispersal flight of aquatic insects distracted synergistically by light intensity and reflection polarization. Naturwissenschaften 101 (5): 385–395.
Bolton, D., M. Mayer-Pinto, G.F. Clark, K.A. Dafforn, W.A. Brassil, A. Becker, and E.L. Johnston. 2017. Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Science of the Total Environment 576: 1–9.
Borges, R., I. Khan, W.E. Johnson, M.T.P. Gilbert, G.J. Zhang, E.D. Jarvis, S.J. O'Brien, and A. Antunes. 2015. Gene loss, adaptive evolution and the co-evolution of plumage coloration genes with opsins in birds. BMC Genomics 16 (1).
Boughman, J.W. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411 (6840): 944–948.
Bradbury, I.R., K. Gardiner, P.V.R. Snelgrove, S.E. Campana, P. Bentzen, and L. Guan. 2006. Larval transport, vertical distributions and localized recruitment in anadromous rainbow smelt (Osmerus mordax). Canadian Journal of Fisheries and Aquatic Sciences 63 (12): 2822–2836.
Brady, P.C., A.A. Gilerson, G.W. Kattawar, J.M. Sullivan, M.S. Twardowski, H.M. Dierssen, M. Gao, K. Travis, R.I. Etheredge, A. Tonizzo, A. Ibrahim, C. Carrizo, Y.L. Gu, B.J. Russell, K. Mislinski, S.L. Zhao, and M.E. Cummings. 2015. Open-ocean fish reveal an omnidirectional solution to camouflage in polarized environments. Science 350 (6263): 965–969.
Brittain, J.E., and T.J. Eikeland. 1988. Invertebrate drift—a review. Hydrobiologia 166 (1): 77–93.
Britto, V.O., and L. Bugoni. 2015. The contrasting feeding ecology of great egrets and roseate spoonbills in limnetic and estuarine colonies. Hydrobiologia 744 (1): 187–210.
Brown, J.S., J.W. Laundre, and M. Gurung. 1999. The ecology of fear: Optimal foraging, game theory, and trophic interactions. Journal of Mammalogy 80 (2): 385–399.
Brüning, A., F. Hölker, S. Franke, T. Preuer, and W. Kloas. 2015. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Science of the Total Environment 511: 516–522.
Brüning, A., F. Hölker, S. Franke, W. Kleiner, and W. Kloas. 2016. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Science of the Total Environment 543: 214–222.
Burford, M.A., I.T. Webster, A.T. Revill, R.A. Kenyon, M. Whittle, and G. Curwen. 2012. Controls on phytoplankton productivity in a wet-dry tropical estuary. Estuarine, Coastal and Shelf Science 113: 141–151.
Butler, K.R. 1998. Coastal protection of sea turtles in Florida. Journal of Land Use & Environmental Law 13: 399–441.
California Lighting Technology Center. 2014. Outdoor lighting retrofits: A guide for the National Park Service and other federal agencies. Davis: Regents of the University of California.
Cannizzaro, J.P., P.R. Carlson, L.A. Yarbro, and C.M. Hu. 2013. Optical variability along a river plume gradient: Implications for management and remote sensing. Estuarine, Coastal and Shelf Science 131: 149–161.
Cao, W.X., Y.Z. Yang, X.Q. Xu, L.M. Huang, and J.L. Zhang. 2003. Regional patterns of particulate spectral absorption in the Pearl River estuary. Chinese Science Bulletin 48 (21): 2344–2351.
Carleton, K. 2009. Cichlid fish visual systems: Mechanisms of spectral tuning. Integrative Zoology 4 (1): 75–86.
Chen, Z.Q., P.H. Doering, M. Ashton, and B.A. Orlando. 2015. Mixing behavior of colored dissolved organic matter and its potential ecological implication in the Caloosahatchee River estuary, Florida. Estuaries and Coasts 38 (5): 1706–1718.
Chew, L.L., V.C. Chong, A.L. Ooi, and A. Sasekumar. 2015. Vertical migration and positioning behavior of copepods in a mangrove estuary: Interactions between tidal, diel light and lunar cycles. Estuarine, Coastal and Shelf Science 152: 142–152.
Cloern, J.E. 1987. Turbidity as a control on phytoplankton biomass and productivity in estuaries. Continental Shelf Research 7 (11-12): 1367–1381.
Cloern, J.E., S.Q. Foster, and A.E. Kleckner. 2014. Phytoplankton primary production in the world's estuarine-coastal ecosystems. Biogeosciences 11 (9): 2477–2501.
Cooke, S.J., L. Sack, C.E. Franklin, A.P. Farrell, J. Beardall, M. Wikelski, and S.L. Chown. 2013. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conservation Physiology 1 (1): cot001.
Cooke, S.J., K.R. Hultine, J.L. Rummer, and C.E. Franklin. 2017. Reflections and progress in conservation physiology. Conservation Physiology 5 (1): cow071.
Cronin, T.W., and J. Marshall. 2011. Patterns and properties of polarized light in air and water. Philosophical Transactions of the Royal Society, B: Biological Sciences 366 (1565): 619–626.
Cronin, T.W., S. Johnsen, N.J. Marshall, and E.J. Warrant. 2014. Visual ecology. Princeton: Princeton University Press.
Csabai, Z., P. Boda, B. Bernath, G. Kriska, and G. Horvath. 2006. A ‘polarisation sun-dial’ dictates the optimal time of day for dispersal by flying aquatic insects. Freshwater Biology 51 (7): 1341–1350.
Dantzer, B., Q.E. Fletcher, R. Boonstra, and M.J. Sheriff. 2014. Measures of physiological stress: A transparent or opaque window into the status, management and conservation of species? Conservation Physiology 2 (1).
Davies, T.W., and T. Smyth. 2018. Why artificial light at night should be a focus for global change research in the 21st century. Global Change Biology 24 (3): 872–882. https://doi.org/10.1111/gcb.13927.
Davies, T.W., J. Bennie, and K.J. Gaston. 2012. Street lighting changes the composition of invertebrate communities. Biology Letters 8 (5): 764–767.
Davies, T.W., J. Bennie, R. Inger, N.H. De Ibarra, and K.J. Gaston. 2013. Artificial light pollution: Are shifting spectral signatures changing the balance of species interactions? Global Change Biology 19 (5): 1417–1423.
Davies, T.W., J.P. Duffy, J. Bennie, and K.J. Gaston. 2014. The nature, extent, and ecological implications of marine light pollution. Frontiers in Ecology and the Environment 12 (6): 347–355.
Davies, T.W., J.P. Duffy, J. Bennie, and K.J. Gaston. 2016. Stemming the tide of light pollution encroaching into marine protected areas. Conservation Letters 9 (3): 164–171.
Day, J., J. Baker, H. Schofield, F. Mathews, and K.J. Gaston. 2015. Part-night lighting and bats. Animal Conservation 18 (6): 512–516.
de Jong, M., L. Jeninga, J.Q. Ouyang, K. van Oers, K. Spoelstra, and M.E. Visser. 2016. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiology & Behavior 155: 172–179.
Depledge, M.H., C.A.J. Godard-Codding, and R.E. Bowen. 2010. Light pollution in the sea. Marine Pollution Bulletin 60 (9): 1383–1385.
Dominoni, D.M. 2015. The effects of light pollution on biological rhythms of birds: An integrated, mechanistic perspective. Journal of Ornithology 156: S409–S418.
Dominoni, D.M., E.O. Carmona-Wagner, M. Hofmann, B. Kranstauber, and J. Partecke. 2014. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. Journal of Animal Ecology 83 (3): 681–692.
Duffy, J.E., J. Paul Richardson, and K.E. France. 2005. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecology Letters 8 (3): 301–309.
Dwyer, R.G., S. Bearhop, H.A. Campbell, and D.M. Bryant. 2013. Shedding light on light: Benefits of anthropogenic illumination to a nocturnally foraging shorebird. Journal of Animal Ecology 82 (2): 478–485.
Endler, J.A. 1990. On the measurement and classification of colour in studies of animal colour patterns. Biological Journal of the Linnean Society 41 (4): 315–352.
Endler, J.A. 1993. The color of light in forests and its implications. Ecological Monographs 63 (1): 1–27.
Epifanio, C.E., and J.H. Cohen. 2016. Behavioral adaptations in larvae of brachyuran crabs: A review. Journal of Experimental Marine Biology and Ecology 482: 85–105.
Erren, T.C., M. Erren, A. Lerchl, and V.B. Meyer-Rochow. 2008. Clockwork blue: On the evolution of non-image-forming retinal photoreceptors in marine and terrestrial vertebrates. Naturwissenschaften 95 (4): 273–279.
Evans Ogden, L.J. 1996. Collision course: The hazards of lighted structures and windows to migrating birds. Toronto: World Wildlife Fund Canada and the Fatal Light Awareness Program.
Falcy, M.R., and B.J. Danielson. 2013. A complex relationship between moonlight and temperature on the foraging behavior of the Alabama beach mouse. Ecology 94 (11): 2632–2637.
Flamarique, I.N., and H.I. Browman. 2000. Wavelength-dependent polarization orientation in Daphnia. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology 186 (11): 1073–1087.
Flecker, A.S. 1992. Fish predation and the evolution of invertebrate drift periodicity—evidence from neotropical streams. Ecology 73 (2): 438–448.
Foster, J.G., D.A. Algera, J.W. Brownscombe, A.J. Zolderdo, and S.J. Cooke. 2016. Consequences of different types of littoral zone light pollution on the parental care behaviour of a freshwater teleost fish. Water, Air, & Soil Pollution 227 (11): 404.
Fukui, D.A.I., M. Murakami, S. Nakano, and T. Aoi. 2006. Effect of emergent aquatic insects on bat foraging in a riparian forest. Journal of Animal Ecology 75 (6): 1252–1258.
Gal, G., E.R. Loew, L.G. Rudstam, and A.M. Mohammadian. 1999. Light and diel vertical migration: Spectral sensitivity and light avoidance by Mysis relicta. Canadian Journal of Fisheries and Aquatic Sciences 56 (2): 311–322.
Gaston, K.J., T.W. Davies, J. Bennie, and J. Hopkins. 2012. Reducing the ecological consequences of night-time light pollution: Options and developments. Journal of Applied Ecology 49 (6): 1256–1266.
Gaston, K. J., M. E. Visser, F. Holker, 2015. The biological impacts of artificial light at night: the research challenge. Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1667):20140133-20140133
Gaston, K.J., J. Bennie, T.W. Davies, and J. Hopkins. 2013. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biological Reviews 88 (4): 912–927.
Glibert, J.P., A.I. Dell, J.P. DeLong, and S. Pawar. 2015. Scaling-up trait variation from individuals to ecosystems. Pages 1–17. In Advances in Ecological Research. Volume 52.
Green, M.C., and P.L. Leberg. 2005. Influence of plumage colour on prey response: Does habitat alter heron crypsis to prey? Animal Behaviour 70 (5): 1203–1208.
Greif, S., I. Borissov, Y. Yovel, and R.A. Holland. 2014. A functional role of the sky's polarization pattern for orientation in the greater mouse-eared bat. Nature Communications 5: 4.
Grubisic, M. 2018. Waters under artificial lights: Does light pollution matter for aquatic primary producers? Limnology and Oceanography Bulletin 27 (3): 76–81.
Grubisic, M., G. Singer, M.C. Bruno, R.H.A. van Grunsven, A. Manfrin, M.T. Monaghan, and F. Hölker. 2017. Artificial light at night decreases biomass and alters community composition of benthic primary producers in a sub-alpine stream. Limnology and Oceanography 62 (6): 2799–2810.
Hampton, S.E., and I.C. Duggan. 2003. Diel habitat shifts of macrofauna in a fishless pond. Marine and Freshwater Research 54 (7): 797–805.
Haney, J.F. 1993. Environmental control of diel vertical migration behaviour. Ergebnisse der Limnologie 39: 1–17.
Harding, L.W., B.W. Meeson, and T.R. Fisher. 1986. Phytoplankton production in two east coast estuaries: Photosynthesis-light functions and patterns of carbon assimilation in Chesapeake and Delaware bays. Estuarine, Coastal and Shelf Science 23 (6): 773–806.
Hart, N.S. 2001a. Variations in cone photoreceptor abundance and the visual ecology of birds. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology 187 (9): 685–697.
Hart, N.S. 2001b. The visual ecology of avian photoreceptors. Progress in Retinal and Eye Research 20 (5): 675–703.
Hastad, O., J.C. Partridge, and A. Odeen. 2009. Ultraviolet photopigment sensitivity and ocular media transmittance in gulls, with an evolutionary perspective. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology 195: 585–590.
Hawryshyn, C.W. 2010. Ultraviolet polarization vision and visually guided behavior in fishes. Brain, Behavior and Evolution 75 (3): 186–194.
Henley, W.J. 1993. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. Journal of Phycology 29 (6): 729–739.
Henn, M., H. Nichols, Y.X. Zhang, and T.H. Bonner. 2014. Effect of artificial light on the drift of aquatic insects in urban central Texas streams. Journal of Freshwater Ecology 29 (3): 307–318.
Hernandez, S.A., and B.L. Peckarsky. 2014. Do stream mayflies exhibit trade-offs between food acquisition and predator avoidance behaviors? Freshwater Science 33 (1): 124–133.
Hölker, F., T. Moss, B. Griefahn, W. Kloas, C.C. Voigt, D. Henckel, A. Hänel, P.M. Kappeler, S. Völker, A. Schwope, S. Franke, D. Uhrlandt, J. Fischer, R. Klenke, C. Wolter, and K. Tockner. 2010a. The dark side of light: A transdisciplinary research agenda for light pollution policy. Ecology and Society 15: 11.
Hölker, F., C. Wolter, E.K. Perkin, and K. Tockner. 2010b. Light pollution as a biodiversity threat. Trends in Ecology & Evolution 25 (12): 681–682.
Hölker, F., C. Wurzbacher, C. Weissenborn, M.T. Monaghan, S.I.J. Holzhauer, and K. Premke. 2015. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Philosophical Transactions of the Royal Society, B: Biological Sciences 370: 10.
Holzhauer, S.I.J., S. Franke, C.C.M. Kyba, A. Manfrin, R. Klenke, C.C. Voigt, D. Lewanzik, M. Oehlert, M.T. Monaghan, S. Schneider, S. Heller, H. Kuechly, A. Bruning, A.C. Honnen, and F. Hölker. 2015. Out of the dark: Establishing a large-scale field experiment to assess the effects of artificial light at night on species and food webs. Sustainability 7 (11): 15593–15616.
Hooper, D.U., E.C. Adair, B.J. Cardinale, J.E.K. Byrnes, B.A. Hungate, K.L. Matulich, A. Gonzalez, J.E. Duffy, L. Gamfeldt, and M.I. O’Connor. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486 (7401): 105–108.
Horváth, G., G. Kriska, P. Malik, and B. Robertson. 2009. Polarized light pollution: A new kind of ecological photopollution. Frontiers in Ecology and the Environment 7 (6): 317–325.
Hunt, D.M., L.S. Carvalho, J.A. Cowing, and W.L. Davies. 2009. Evolution and spectral tuning of visual pigments in birds and mammals. Philosophical Transactions of the Royal Society, B: Biological Sciences 364 (1531): 2941–2955.
Johnsen, S., A. Kelber, E. Warrant, A.M. Sweeney, E.A. Widder, R.L. Lee, and J. Hernandez-Andres. 2006. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. Journal of Experimental Biology 209 (5): 789–800.
Jung, K., and E.K.V. Kalko. 2010. Where forest meets urbanization: Foraging plasticity of aerial insectivorous bats in an anthropogenically altered environment. Journal of Mammalogy 91 (1): 144–153.
Kamermans, M., and C. Hawryshyn. 2011. Teleost polarization vision: How it might work and what it might be good for. Philosophical Transactions of the Royal Society, B: Biological Sciences 366 (1565): 742–756.
Kautza, A., and S.M.P. Sullivan. 2016. The energetic contributions of aquatic primary producers to terrestrial food webs in a mid-size river system. Ecology 97: 694–705.
Kempenaers, B., P. Borgström, P. Loës, E. Schlicht, and M. Valcu. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Current Biology 20 (19): 1735–1739.
Kirk, J.T. 2011. Light and photosynthesis in aquatic ecosystems. 3rd ed. Cambridge: Cambridge University Press.
Koyanagi, M., T. Nagata, K. Katoh, S. Yamashita, and F. Tokunaga. 2008. Molecular evolution of arthropod color vision deduced from multiple opsin genes of jumping spiders. Journal of Molecular Evolution 66 (2): 130–137.
Kronfeld-Schor, N., and T. Dayan. 2003. Partitioning of time as an ecological resource. Annual Review of Ecology, Evolution, and Systematics 34 (1): 153–181.
Kronfeld-Schor, N., D. Dominoni, H. de la Iglesia, O. Levy, E.D. Herzog, T. Dayan, and C. Helfrich-Forster. 2013. Chronobiology by moonlight. Proceedings of the Royal Society B: Biological Sciences 280: 11.
Kuijper, D.P.J., J. Schut, D. van Dullemen, H. Toorman, N. Goossens, J. Ouwehand, and H.J.G.A. Limpens. 2008. Experimental evidence of light disturbance along the commuting routes of pond bats (Myotis dasycneme). Lutra 51: 37–49.
Kurvers, R., and F. Hölker. 2015. Bright nights and social interactions: A neglected issue. Behavioral Ecology 26 (2): 334–339.
Kurvers, R.H.J.M., J. Drägestein, F. Hölker, A. Jechow, J. Krause, and D. Bierbach. 2018. Artificial light at night affects emergence from a refuge and space use in guppies. Scientific Reports 8 (1): 14131.
Kyba, C.C.M., T. Ruhtz, J. Fischer, and F. Hölker. 2011. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS One 6: 9.
Lamb, T.D. 2013. Evolution of phototransduction, vertebrate photoreceptors and retina. Progress in Retinal and Eye Research 36: 52–119.
Lamb, T.D., S.P. Collin, and E.N. Pugh. 2007. Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nature Reviews Neuroscience 8 (12): 960–975.
Le Duc, D., and T. Schöneberg. 2016. Adaptation to nocturnality–learning from avian genomes. BioEssays 38 (7): 694–703.
Levin, S.A. 1992. The problem of pattern and scale in ecology: The Robert H. MacArthur award lecture. Ecology 73 (6): 1943–1967.
Levin, L.A., D.F. Boesch, A. Covich, C. Dahm, C. Erseus, K.C. Ewel, R.T. Kneib, A. Moldenke, M.A. Palmer, P. Snelgrove, D. Strayer, and J.M. Weslawski. 2001. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4 (5): 430–451.
Lewanzik, D., and C.C. Voigt. 2014. Artificial light puts ecosystem services of frugivorous bats at risk. Journal of Applied Ecology 51 (2): 388–394.
Ley, J.A., and I.A. Halliday. 2007. Diel variation in mangrove fish abundances and trophic guilds of northeastern Australian estuaries with a proposed trophodynamic model. Bulletin of Marine Science 80: 681–720.
Link, J.S., W.T. Stockhausen, and E.T. Methratta. 2005. Food-web theory in marine ecosystems. In Aquatic food webs: an ecosystem approach, 98–114. Oxford: Oxford University Press.
Loew, E.R., and W.N. McFarland. 1990. The underwater visual environment. In The visual system of fish, 1–43. Dordrecht: Springer Netherlands.
Longcore, T., and C. Rich. 2004. Ecological light pollution. Frontiers in Ecology and the Environment 2 (4): 191–198.
Loss, S.R., T. Will, S.S. Loss, and P.P. Marra. 2014. Bird–building collisions in the United States: Estimates of annual mortality and species vulnerability. The Condor 116 (1): 8–23.
Lythgoe, J.N. 1968. Visual pigments and visual range underwater. Vision Research 8 (8): 997–1011.
Lythgoe, J.N. 1979. Ecology of vision. Oxford: Clarendon Press.
Lyytimäki, J. 2013. Nature’s nocturnal services: Light pollution as a non-recognised challenge for ecosystem services research and management. Ecosystem Services 3: e44–e48.
Madliger, C.L. 2012. Toward improved conservation management: A consideration of sensory ecology. Biodiversity and Conservation 21 (13): 3277–3286.
Manfrin, A. 2017. Effects of artificial light at night (ALAN) on interactions between aquatic and terrestrial ecosystems. PhD dissertation, Freie Universität Berlin.
Manfrin, A., G. Singer, S. Larsen, N. Weiß, R.H.A. van Grunsven, N.S. Weiß, S. Wohlfahrt, M.T. Monaghan, and F. Hölker. 2017. Artificial light at night affects organism flux across ecosystem boundaries and drives community structure in the recipient ecosystem. Frontiers in Environmental Science 5(61): 1–14.
McCauley, D.J., E. Hoffmann, H.S. Young, and F. Micheli. 2012. Night shift: Expansion of temporal niche use following reductions in predator density. PLoS One 7 (6): e38871.
McComb, D.M., S.M. Kajiura, A.Z. Horodysky, and T.M. Frank. 2013. Comparative visual function in predatory fishes from the Indian River Lagoon. Physiological and Biochemical Zoology 86 (3): 285–297.
McFarland, W.N., and C.M. Wahl. 1996. Visual constraints on migration behavior of juvenile French grunts. Environmental Biology of Fishes 46 (2): 109–122.
McFarland, W.N., J.C. Ogden, and J.N. Lythgoe. 1979. The influence of light on the twilight migrations of grunts. Environmental Biology of Fishes 4 (1): 9–22.
McLaren, J.D., J.J. Buler, T. Schreckengost, J.A. Smolinsky, M. Boone, E. Emiel van Loon, D.K. Dawson, and E.L. Walters. 2018. Artificial light at night confounds broad-scale habitat use by migrating birds. Ecology Letters 21 (3): 356–364.
McNeil, R., and J.R. Rodríguez. 1996. Nocturnal foraging in shorebirds. International Wader Studies 8: 114–121.
McNeil, R., P. Drapeau, and J.D. Gosscustard. 1992. The occurrence and adaptive significance of nocturnal habits in waterfowl. Biological Reviews of the Cambridge Philosophical Society 67 (4): 381–419.
McNeil, R., L.M. Rojas, G. Marín, and Y.M.R. Figueroa. 2004. Actividad nocturna y visión en aves playeras de la región Neotropical. Ornotología Neotropical 15: 223–232.
Meyer, L.A., and S.M.P. Sullivan. 2013. Bright lights, big city: Influences of ecological light pollution on reciprocal stream-riparian invertebrate fluxes. Ecological Applications 23 (6): 1322–1330.
Migaud, H., M. Cowan, J. Taylor, and H.W. Ferguson. 2007. The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar. Aquaculture 270 (1–4):390–404.
Miyasaka, H., and S. Nakano. 2001. Drift dispersal of mayfly nymphs in the presence of chemical and visual cues from diurnal drift- and nocturnal benthic-foraging fishes. Freshwater Biology 46 (9): 1229–1237.
Moore, M.V., S.M. Pierce, H.M. Walsh, S.K. Kvalvik, and J.D. Lim. 1998. Urban light pollution alters the diel vertical migration of Daphnia. SIL Proceedings 1922–2010 (27): 779–782.
Mueller, R.P., and M.A. Simmons. 2008. Characterization of gatewell orifice lighting at the Bonneville Dam second powerhouse and compendium of research on light guidance with juvenile salmonids, 1–55. Richland: Pacific Northwest National Laboratory.
Muheim, R. 2011. Behavioural and physiological mechanisms of polarized light sensitivity in birds. Philosophical Transactions of the Royal Society, B: Biological Sciences 366 (1565): 763–771.
Munz, F.W., and W.N. McFarland. 1973. The significance of spectral position in the rhodopsins of tropical marine fishes. Vision Research 13 (10): 1829–IN1821.
Nagelkerken, I. 2009. Ecological connectivity among tropical coastal ecosystems, introduction. In Ecological connectivity among tropical coastal ecosystems, 1–6. Netherlands: Springer.
Nagelkerken, I., M. Dorenbosch, W.C.E.P. Verberk, E.C. De La Morinière, and G. Van Der Velde. 2000. Day-night shifts of fishes between shallow-water biotopes of a Caribbean bay, with emphasis on the nocturnal feeding of Haemulidae and Lutjanidae. Marine Ecology Progress Series 194: 55–64.
Navara, K.J., and R.J. Nelson. 2007. The dark side of light at night: Physiological, epidemiological, and ecological consequences. Journal of Pineal Research 43 (3): 215–224.
Navarro-Barranco, C., and L.E. Hughes. 2015. Effects of light pollution on the emergent fauna of shallow marine ecosystems: Amphipods as a case study. Marine Pollution Bulletin 94 (1-2): 235–240.
Naylor, E. 2006. Orientation and navigation in coastal and estuarine zooplankton. Marine and Freshwater Behaviour and Physiology 39 (1): 13–24.
Nightingale, B., T. Longcore, and C.A. Simenstad. 2006. Artificial night lighting and fishes. In Ecological consequences of artificial night lighting, 257–276. Washington: Island Press.
Norcross, B.L., and R.F. Shaw. 1984. Oceanic and estuarine transport of fish eggs and larvae: A review. Transactions of the American Fisheries Society 113 (2): 153–165.
Ouyang, J.Q., S. Davies, and D. Dominoni. 2018. Hormonally mediated effects of artificial light at night on behavior and fitness: Linking endocrine mechanisms with function. Journal of Experimental Biology 221 (6): jeb156893.
Ouyang, J.Q., M. de Jong, M. Hau, M.E. Visser, R.H.A. van Grunsven, and K. Spoelstra. 2015 Stressful colours: corticosterone concentrations in a free-living songbird vary with the spectral composition of experimental illumination. Biology Letters 11 (8):20150517.
Paetzold, A., C.J. Schubert, and K. Tockner. 2005. Aquatic terrestrial linkages along a braided-river: Riparian arthropods feeding on aquatic insects. Ecosystems 8 (7): 748–759.
Palmer, M.A., J.D. Allan, and C.A. Butman. 1996. Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends in Ecology & Evolution 11 (8): 322–326.
Peckarsky, B.L., and A.R. McIntosh. 1998. Fitness and community consequences of avoiding multiple predators. Oecologia 113 (4): 565–576.
Perkin, E.K., F. Hölker, J.S. Richardson, J.P. Sadler, C. Wolter, and K. Tockner. 2011. The influence of artificial light on stream and riparian ecosystems: Questions, challenges, and perspectives. Ecosphere 2: 122.
Perkin, E.K., F. Hölker, and K. Tockner. 2014a. The effects of artificial lighting on adult aquatic and terrestrial insects. Freshwater Biology 59 (2): 368–377.
Perkin, E.K., F. Hölker, K. Tockner, and J.S. Richardson. 2014b. Artificial light as a disturbance to light-naive streams. Freshwater Biology 59 (11): 2235–2244.
Pignatelli, V., S.E. Temple, T.H. Chiou, N.W. Roberts, S.P. Collin, and N.J. Marshall. 2011. Behavioural relevance of polarization sensitivity as a target detection mechanism in cephalopods and fishes. Philosophical Transactions of the Royal Society, B: Biological Sciences 366 (1565): 734–741.
Quinn, T.P. 2004. The behavior and ecology of Pacific salmon and trout. Seattle: University of Washington Press.
Radabaugh, K.R., E.M. Malkin, D.J. Hollander, and E.B. Peebles. 2014. Evidence for light-environment control of carbon isotope fractionation by benthic microalgal communities. Marine Ecology Progress Series 495: 77–90.
Ramirez-Martinez, G.A., G.A. Castellanos-Galindo, and U. Krumme. 2016. Tidal and diel patterns in abundance and feeding of a marine-estuarine-dependent fish from macrotidal mangrove creeks in the Tropical Eastern Pacific (Colombia). Estuaries and Coasts 39 (4): 1249–1261.
Rich, C., and T. Longcore. 2006. Introduction. In Ecological Consequences of Artificial Night Lighting, ed. C. Rich and T. Longcore, 1–13. Washington: Island Press.
Riley, W.D., B. Bendall, M.J. Ives, N.J. Edmonds, and D.L. Maxwell. 2012. Street lighting disrupts the diel migratory pattern of wild Atlantic salmon, Salmo salar L., smolts leaving their natal stream. Aquaculture 330-333: 74–81.
Riley, W.D., P.I. Davison, D.L. Maxwell, and B. Bendall. 2013. Street lighting delays and disrupts the dispersal of Atlantic salmon (Salmo salar) fry. Biological Conservation 158: 140–146.
Riley, W.D., P.I. Davison, D.L. Maxwell, R.C. Newman, and M.J. Ives. 2015. A laboratory experiment to determine the dispersal response of Atlantic salmon (Salmo salar) fry to street light intensity. Freshwater Biology 60 (5): 1016–1028.
Ringelberg, J. 1999. The photobehaviour of Daphnia spp. as a model to explain diel vertical migration in zooplankton. Biological Reviews 74 (4): 397–423.
Robertson, B., G. Kriska, V. Horvath, and G. Horvath. 2010. Glass buildings as bird feeders: Urban birds exploit insects trapped by polarized light pollution. Acta Zoologica Academiae Scientiarum Hungaricae 56: 283–293.
Robinson, E., A.R. Jerrett, S.E. Black, and W. Davison. 2011. Visual acuity of snapper Pagrus auratus: Effect of size and spectral composition. Journal of Fish Biology 79 (7): 1883–1894.
Rodriguez, A., N.D. Holmes, P.G. Ryan, K.J. Wilson, L. Faulquier, Y. Murillo, A.F. Raine, J.F. Penniman, V. Neves, B. Rodriguez, J.J. Negro, A. Chiaradia, P. Dann, T. Anderson, B. Metzger, M. Shirai, L. Deppe, J. Wheeler, P. Hodum, C. Gouveia, V. Carmo, G.P. Carreira, L. Delgado-Alburqueque, C. Guerra-Correa, F.X. Couzi, M. Travers, and M. Le Corre. 2017. Seabird mortality induced by land-based artificial lights. Conservation Biology 31 (5): 986–1001.
Rojas de Azuaje, L.M., S. Tai, and R. McNeil. 1993. Comparison of rod/cone ratio in three species of shorebirds having different nocturnal foraging strategies. The Auk: 141–145.
Rojas, L.M., R. McNeil, T. Cabana, and P. Lachapelle. 1999a. Behavioral, morphological and physiological correlates of diurnal and nocturnal vision in selected wading bird species. Brain, Behavior and Evolution 53 (5-6): 227–242.
Rojas, L.M., R. McNeil, T. Cabana, and P. Lachapelle. 1999b. Diurnal and nocturnal visual capabilities in shorebirds as a function of their feeding strategies. Brain, Behavior and Evolution 53 (1): 29–43.
Rosenblatt, A.E., M.R. Heithaus, F.J. Mazzotti, M. Cherkiss, and B.M. Jeffery. 2013. Intra-population variation in activity ranges, diel patterns, movement rates, and habitat use of American alligators in a subtropical estuary. Estuarine, Coastal and Shelf Science 135: 182–190.
Rotics, S., T. Dayan, and N. Kronfeld-Schor. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. Journal of Mammalogy 92 (1): 159–168.
Royal Commission on Environmental Pollution (RCEP). 2009. Artificial Light in the Environment: The Royal Commission on Environmental Pollution.
Rydell, J. 1992. Exploitation of insects around streetlamps by bats in Sweden. Functional Ecology 6 (6): 744–750.
Sabbah, S., M.F. Habib-Nayany, Z. Dargaei, F.E. Hauser, M. Kamermans, and C.W. Hawryshyn. 2013. Retinal region of polarization sensitivity switches during ontogeny of rainbow trout. Journal of Neuroscience 33 (17): 7428–7438.
Sabo, J.L., J.C. Finlay, T. Kennedy, and D.M. Post. 2010. The role of discharge variation in scaling of drainage area and food chain length in rivers. Science 330 (6006): 965–967.
Saint-Béat, B., D. Baird, H. Asmus, R. Asmus, C. Bacher, S.R. Pacella, G.A. Johnson, V. David, A.F. Vézina, and N. Niquil. 2015. Trophic networks: How do theories link ecosystem structure and functioning to stability properties? A review. Ecological Indicators 52: 458–471.
Sanders, D., and K.J. Gaston. 2018. How ecological communities respond to artificial light at night. Journal of Experimental Zoology 2018: 1–7. https://doi.org/10.1002/jez.2157.
Santos, C.D., A.C. Miranda, J.P. Granadeiro, P.M. Lourenco, S. Saraiva, and J.M. Palmeirim. 2010. Effects of artificial illumination on the nocturnal foraging of waders. Acta Oecologica-International Journal of Ecology 36 (2): 166–172.
Scheuerell, M.D., and D.E. Schindler. 2003. Diel vertical migration by juvenile sockeye salmon: Empirical evidence for the antipredation window. Ecology 84 (7): 1713–1720.
Schmitz, L., and P.C. Wainwright. 2011. Nocturnality constrains morphological and functional diversity in the eyes of reef fishes. BMC Evolutionary Biology 11 (1): 338.
Schroer, S., and F. Hölker. 2017. Light pollution reduction. In Handbook of advanced lighting technology, ed. R. Karlicek, C.C. Sun, G. Zissis, and R. Ma. Switzerland: Springer International Publishing.
Schwind, R. 1995. Spectral regions in which aquatic insects see reflected polarized light. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology 177: 439–448.
Seehausen, O., J.J.M. Van Alphen, and F. Witte. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277 (5333): 1808–1811.
Seehausen, O., Y. Terai, I.S. Magalhaes, K.L. Carleton, H.D.J. Mrosso, R. Miyagi, I. van der Sluijs, M.V. Schneider, M.E. Maan, and H. Tachida. 2008. Speciation through sensory drive in cichlid fish. Nature 455 (7213): 620–626.
Shashar, N., R. Hagan, J.G. Boal, and R.T. Hanlon. 2000. Cuttlefish use polarization sensitivity in predation on silvery fish. Vision Research 40 (1): 71–75.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2015. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries and Coasts 38 (2): 401–414.
Shichida, Y., and T. Matsuyama. 2009. Evolution of opsins and phototransduction. Philosophical Transactions of the Royal Society, B: Biological Sciences 364 (1531): 2881–2895.
Small, C., and R.J. Nicholls. 2003. A global analysis of human settlement in coastal zones. Journal of Coastal Research 19: 584–599.
Stanley, M.C., J.R. Beggs, I.E. Bassett, B.R. Burns, K.N. Dirks, D.N. Jones, W.L. Linklater, C. Macinnis-Ng, R. Simcock, G. Souter-Brown, S.A. Trowsdale, and K.J. Gaston. 2015. Emerging threats in urban ecosystems: A horizon scanning exercise. Frontiers in Ecology and the Environment 13 (10): 553–560.
Stich, D.S., G.B. Zydlewski, J.F. Kocik, and J.D. Zydlewski. 2015. Linking behavior, physiology, and survival of Atlantic salmon smolts during estuary migration. Marine and Coastal Fisheries 7 (1): 68–86.
Sullivan, S.M.P., and A.D. Rodewald. 2012. In a state of flux: The energetic pathways that move contaminants from aquatic to terrestrial environments. Environmental Toxicology and Chemistry 31 (6): 1175–1183.
Sullivan, S.M.P., K. Hossler, and L.A. Meyer. in press. Artificial lighting at night alters aquatic-riparian invertebrate food webs. Ecological Applications.
Tarlow, E.M., M. Hau, D.J. Anderson, and M. Wikelski. 2003. Diel changes in plasma melatonin and corticosterone concentrations in tropical Nazca boobies (Sula granti) in relation to moon phase and age. General and Comparative Endocrinology 133 (3): 297–304.
Thomas, R.J., T. Szekely, R.F. Powell, and I.C. Cuthill. 2006. Eye size, foraging methods and the timing of foraging in shorebirds. Functional Ecology 20 (1): 157–165.
Thomas, J.R., J. James, R.C. Newman, W.D. Riley, S.W. Griffiths, and J. Cable. 2016. The impact of streetlights on an aquatic invasive species: Artificial light at night alters signal crayfish behaviour. Applied Animal Behaviour Science 176: 143–149.
Thompson, R.M., U. Brose, J.A. Dunne, R.O. Hall, S. Hladyz, R.L. Kitching, N.D. Martinez, H. Rantala, T.N. Romanuk, and D.B. Stouffer. 2012. Food webs: Reconciling the structure and function of biodiversity. Trends in Ecology & Evolution 27 (12): 689–697.
Toyama, M., M. Hironaka, Y. Yamahama, H. Horiguchi, O. Tsukada, N. Uto, Y. Ueno, F. Tokunaga, K. Seno, and T. Hariyama. 2008. Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species—A qualitative and comparative study. Photochemistry and Photobiology 84 (4): 996–1002.
Van der Sluijs, I., S.M. Gray, M.C.P. Amorim, I. Barber, U. Candolin, A.P. Hendry, R. Krahe, M.E. Maan, A.C. Utne-Palm, H.J. Wagner, and B.B. Wong. 2011. Communication in troubled waters: Responses of fish communication systems to changing environments. Evolutionary Ecology 25 (3): 623–640.
Veilleux, C.C., and M.E. Cummings. 2012. Nocturnal light environments and species ecology: Implications for nocturnal color vision in forests. Journal of Experimental Biology 215 (23): 4085–4096.
Verovnik, R., Ž. Fišer, and V. Zakšek. 2015. How to reduce the impact of artificial lighting on moths: A case study on cultural heritage sites in Slovenia. Journal of Nature Conservation 28: 105–111.
Viets, K., K.C. Eldred, and R.J. Johnston. 2016. Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends in Genetics 32 (10): 638–659.
Voigt, C.C., K. Rehnig, O. Lindecke, and G. Pētersons. 2018. Migratory bats are attracted by red light but not by warm-white light: Implications for the protection of nocturnal migrants. Ecology and Evolution 2018 (00): 1–9.
Wald, G. 1935. Pigments of the bull frog retina. Nature 136: 382.
Wilkinson, E.B., L.C. Branch, and D.L. Miller. 2013. Functional habitat connectivity for beach mice depends on perceived predation risk. Landscape Ecology 28 (3): 547–558.
Williams, D.D., and N.E. Williams. 1998. Seasonal variation, export dynamics and consumption of freshwater invertebrates in an estuarine environment. Estuarine, Coastal and Shelf Science 46 (3): 393–410.
Witherington B.E., and R.E. Martin. 2003. Understanding, assessing, and resolving light-pollution problems on sea turtle nesting beaches. Florida Marine Institute technical reports. Florida Fish and Wildlife Conservation Commission FMRI technical report TR-2.
Yeager, L.A., E.W. Stoner, J.R. Peters, and C.A. Layman. 2016. A terrestrial-aquatic food web subsidy is potentially mediated by multiple predator effects on an arboreal crab. Journal of Experimental Marine Biology and Ecology 475: 73–79.
Yokoyama, S. 2008. Evolution of dim-light and color vision pigments. Annual Review of Genomics and Human Genetics 9 (1): 259–282.
Zapata, M.J., and S.M.P. Sullivan. in press. Spatial and seasonal variability of emergent aquatic insects and nearshore spiders in a subtropical estuary. Marine and Freshwater Research. https://doi.org/10.1071/MF18130.
Zapata, M.J., L.A. Yeager, and C.A. Layman. 2014. Day–night patterns in natural and artificial patch reef fish assemblages of the Bahamas. Caribbean Naturalist 18: 1–15.
Acknowledgements
We extend our thanks to anonymous reviewers for their careful critiques of earlier versions of the manuscript. This research was funded by The Ohio State University Office, School of Environment and Natural Resources and McIntyre-Stennis funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kenneth L. Heck
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zapata, M.J., Sullivan, S.M.P. & Gray, S.M. Artificial Lighting at Night in Estuaries—Implications from Individuals to Ecosystems. Estuaries and Coasts 42, 309–330 (2019). https://doi.org/10.1007/s12237-018-0479-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0479-3