Abstract
Coastal areas provide critical nesting habitat for marine turtles. Understanding how artificial light might impact populations is key to guide management strategies. Here we assess the extent to which nesting populations of four marine turtle species—leatherback (Dermochelys coriacea), olive ridley (Lepidochelys olivacea), hawksbill (Eretmochelys imbricata) and two subpopulations of loggerhead (Caretta caretta) turtles—are exposed to light pollution across 604 km of the Brazilian coast. We used yearly night-time satellite images from two 5-year periods (1992–1996 and 2008–2012) from the US Air Force Defense Meteorological Satellite Programme (DMSP) to determine the proportion of nesting areas that are exposed to detectable levels of artificial light and identify how this has changed over time. Over the monitored time-frame, 63.7% of the nesting beaches experienced an increase in night light levels. Based on nest densities, we identified 54 reproductive hotspots: 62.9% were located in areas potentially exposed to light pollution. Light levels appeared to have a significant effect on nest densities of hawksbills and the northern loggerhead turtle stock, however high nest densities were also seen in lit areas. The status of all species/subpopulations has improved across the time period despite increased light levels. These findings suggest that (1) nest site selection is likely primarily determined by variables other than light and (2) conservation strategies in Brazil appear to have been successful in contributing to reducing impacts on nesting beaches. There is, however, the possibility that light also affects hatchlings in coastal waters, and impacts on population recruitment may take longer to fully manifest in nesting numbers. Recommendations are made to further this work to provide deeper insights into the impacts of anthropogenic light on marine turtles.
Similar content being viewed by others
Introduction
The introduction and proliferation of artificial light at night has transformed the night-time environment over significant portions of our planet’s surface (Longcore and Rich 2004). Light pollution can affect organisms in different ways, impacting their ecosystems and the processes that occur within them. The effects on wildlife can include changes in orientation systems and attraction or repulsion from the altered light environment, which ultimately might have consequences for foraging, reproduction, migration and communication (Longcore and Rich 2004).
Remote sensing has been used to measure artificial night-time light (Cinzano et al. 2001) and its effects on wildlife, including invertebrates, birds, reptiles and mammals (Longcore and Rich 2004; Aubrecht et al. 2008; Kamrowski et al. 2012; Mazor et al. 2013; Weishampel et al. 2016). Worldwide measurements of artificial light have been collected by the US Air Force Defense Meteorological Satellite Program (DMSP) Operational Linescan System (OLS) since 1992 (Elvidge et al. 2007). These data can be downloaded from the NOAA’s National Geophysical Data Center (NGDC) and represent cloud-free composite images created from multiple nightly orbits by the DMSP satellites for each year (Elvidge et al. 2001, 2007).
Light pollution can impact fundamental biological processes for marine turtles. After emerging from nests, hatchlings are generally guided by several cues to find the ocean, including beach elevation and natural brightness (Lorne and Salmon 2007; Pendoley and Kamrowski 2015). The presence of artificial lighting can cause disruption to sea-finding, increasing predation risk and also possibly causing exhaustion, dehydration and the loss of energy that is vital to their initial migration towards the open sea, which could result in higher mortality (Witherington and Bjorndal 1991). Artificial lighting at nesting beaches is also believed to potentially disrupt adult turtle nesting (Pendoley and Kamrowski 2015; Silva et al. 2017) with females probably avoiding particularly lit areas (Salmon 2003). The presence of artificial light at beaches and near shore waters may also alter the predator–prey dynamics, influencing the behaviour of species that predate on sea turtle egg and hatchlings (e.g. Silva et al. 2017), as well as attracting hatchlings dispersing from natal beaches (Thums et al. 2016).
In marine turtles, suitable nesting beaches are determined by several environmental factors that operate at different spatial scales. The range of suitable nesting areas is most likely determined primarily by temperature (Pike 2013). At regional scales, nest density appears to be strongly linked to how well the offshore migrations of hatchling marine turtles is facilitated, that is, coastal areas in close proximity to favourable currents for transporting hatchlings to nursery habitats would have higher nest densities than other less favourable areas (Putman et al. 2010a, b; Okuyama et al. 2011; Shillinger et al. 2012; Putman 2018). At local scales, site-specific topographic features such as vegetation, sand dunes, beach slope, substrate and lighting become increasingly important (Price et al. 2018).
The Brazilian coast and offshore islands hold nesting grounds for five sea turtle species: the loggerhead (Caretta caretta), hawksbill (Eretmochelys imbricata), olive ridley (Lepidochelys olivacea), leatherback (Dermochelys coriacea) and green turtles (Chelonia mydas) (Marcovaldi and Marcovaldi 1999). Conservation efforts started by TAMAR (the Brazilian Sea Turtle Conservation Programme) in 1982 (Marcovaldi and Marcovaldi 1999) have contributed to increasing or stable trends seen in nesting numbers for all the five species (da Silva et al. 2007; Marcovaldi et al. 2007; Marcovaldi and Chaloupka 2007; Bellini et al. 2013; Colman et al. 2019). Despite the possible recovery from past exploitation seen for the marine turtles in Brazil, the persistence of various threats—fisheries bycatch, coastal development, pollution and climate change—means these populations remain subject of conservation concern. Furthermore, in recent years the human population in Brazil has grown considerably, with much of the population growth seen along coastal areas (Instituto Brasileiro de Geografia e Estatística 2013). The consequent coastal development and associated artificial lighting represent a potential threat to marine turtles in Brazil.
Here, we assessed the extent that globally important marine turtle nesting habitats in Brazil are spatially exposed to artificial light at a national scale and how this has changed over time, considering the proportion of nesting marine turtles that are likely to be potentially exposed to coastal light pollution (as assessed using satellite images). We identified reproductive hotspots within Brazilian nesting grounds and assessed what level of exposure to light pollution they potentially experience. We also investigated correlation between light levels and nest densities for each marine turtle species/subpopulation.
Methods
Light data
We downloaded yearly night-time stable lights composite images for two different 5-year periods (1992–1996 and 2008–2012) from the National Geophysical Data Centre (USA; https://ngdc.noaa.gov/eog/dmsp/downloadV4composites.html). They were created with data from the Defense Meteorological Satellite Program (DMSP) Operational Linescan System (OLS) and represent Average Visible, Stable Lights (lights from cities, towns, and other sites with persistent lighting) and Cloud Free Coverage images. These images are nominally at 1 km resolution; however, they are re-sampled from data at an equal angle of approximately 2.7 km resolution at the equator. Each pixel is represented by a digital number (DN) between 0 and 63, with zero representing total darkness and bright-lit areas generally saturating at values of 63. Because there is no on-board calibration of the satellites, the images were inter-calibrated and drift-corrected according to Bennie et al. (2015). This method for cross-calibration included correcting for geolocation errors between the years and posterior intercalibration of the images using a sixth order polynomial regression on the median (for full description of calibration methods see Bennie et al. 2015). Even after being corrected, the annual light data should be interpreted with caution, especially considering the variation in sensitivity among years and the saturation in urban areas. A more precautionary approach is to use average values for 5-year periods. In this way, DMSP/OLS night-time lights remain a valuable source for detecting long-term trends in the distribution of artificial light at night (Bennie et al. 2015). The OLS images cover spectral responses from 440 to 940 nm, having the highest sensitivity within the 500 to 650 nm region (Freitas et al. 2017). These wavelengths are consistent with those believed to disrupt adult and hatchling marine turtles (within the 440 to 700 nm range), and although there are differences in response among species, adults have been shown greater sensitivity to longer wavelengths (approximately 580 nm; Levenson et al. 2004) and hatchlings more sensitivity to shorter wavelengths (from 350 to 540 nm; Witherington and Bjorndal 1991; Witherington and Martin 2000).
Marine turtle nesting areas
The georeferenced location of nesting areas in Brazil were obtained from TAMAR and included areas between latitudes 10° 34′ 29″ S and 22° 05′ 32″ S (n = 604 ~ 1 km segments). The coast of four Brazilian states were encompassed, from north to south: Sergipe (SE), Bahia (BA), Espírito Santo (ES) and Rio de Janeiro (RJ) (Fig. 1). The northern part of the area, along the coasts of Sergipe and northern Bahia, represents key nesting areas for the northern loggerhead turtle stock, olive ridley and hawksbill turtles, across 347 km (da Silva et al. 2007; Marcovaldi et al. 2007, 2016; Reis et al. 2010). The southern part of the area holds important nesting grounds for the genetically distinct southern loggerhead turtle stock, across 257 km of the northern coast of the states of Rio de Janeiro and Espírito Santo (Baptistotte et al. 2003; Lima et al. 2012). Leatherback turtles have their only known regular nesting area in Brazil in the northern coast of Espírito Santo, along 159 km (Colman et al. 2019) (Fig. 1). Green turtles have their major nesting areas restricted to the offshore oceanic islands, with only a small number of nests seen per year on continental beaches (Almeida et al. 2011; Bellini et al. 2013), thus this species was not considered in the analysis.
Distribution of turtle nesting. a Map of Brazil with marine turtle nesting areas considered in this study denoted by black lines (inset shows Brazil and study region within the context). b Proportional spatial distribution of nests by species (n = 15 areas in 4 regions). We considered the estimated annual average number of recorded nests per area and species during 2008–2012. Labels: Sergipe (SE), Bahia (BA), Espírito Santo (ES) and Rio de Janeiro (RJ). For C. caretta, BA + SE represent the northern stock (NS) and ES + RJ represent the southern stock (SS)
Nesting data
To determine the proportion of nesting marine turtles that are likely to be potentially exposed to coastal light pollution, we used data on nest counts collected by Projeto TAMAR. During the nesting seasons (September to March), morning patrols were conducted daily to quantify nesting activity from the preceding night, following standard methodology (Marcovaldi and Marcovaldi 1999). We used the average annual nesting numbers per species during the most recent period (5-year average of nest location; 2008–2012) as being representative of current nesting levels and because the monitoring effort over the nesting areas was constant throughout the period (TAMAR unpublished data). Species was determined during nest excavation, after hatchling emergence. As it was not possible to determine species from all nests in the field, we estimated the total number of nests per species laid in each season at each site. The number of observed nests from a given species was added to the number of nests from unknown species (ni), multiplied by the ratio of the number of observed nests from a given species to the total number of nests of known species, according to the formula below:
This is based on the assumption that the nests for which the species was known represented a random sample of the nests laid in the station area (Marcovaldi et al. 2007).
Nest densities
Nest density per species/subpopulation was calculated as nests km−1 year−1. An indicator to measure the relative importance of each section of the beach in terms of their nest density was created. As part of TAMAR standard methodology, the entire 604 km of the study area are divided by permanent marker posts at each kilometre. The average annual number of nests per species/subpopulation on each one of the kilometres during the period 2008–2012 (5 years of data) was calculated. Each kilometre of the beach was then classified in terms of nest density as either high, medium or low, by locating the average annual number of nests on that kilometre in comparison with the total ~ 1 km segments (n = 604), relative to each species or subpopulation: high-density sections were those with average annual number of nests in the top 25% of the distribution; low-density sites were those in the lowest 25% of the distribution; and sites with densities in-between the two previous categories were classified as medium-density. A very high-density category was assigned to the top three beach segments for each species/subpopulation (Fuentes et al. 2016). Each species had its own value of density per segment, irrespective if the segment was used by multiple species (i.e. one segment could have both a low nest density for one species and a very high nest density for another).
Processing
We created an average calibrated image for both the first (1992–1996) and the last (2008–2012) 5 years of data, as well as one with the difference or change between them. Pixels were considered potentially exposed to detectable artificial light when they had values higher than 5.5 DN units. Observed changes in brightness were considered when pixels increased or decreased by more than a threshold of 3 DN units of difference between the mean of each of the first and last five years of the surveyed periods. Values of 5.5 and 3 can be used as reliable thresholds for exposure and change in artificial light, considering the noise within the dataset (Bennie et al. 2015; Duffy et al. 2015). We then overlaid the information on nesting areas with the DMSP data for both periods (1992–1996 and 2008–2012). We used the ‘spatial analyst sample tool’ in ArcMap 10 to determine the nearest pixel value associated to each monitored segment (n = 604 ~ 1 km segments). The proportion of nesting areas potentially exposed to artificial light, or changes in brightness, was calculated dividing the number of exposed pixels or pixels with observed change by the total number of pixels within each area. We then compared the two periods (1992–1996 and 2008–2012) to assess potential changes and trends in brightness.
Nest densities and relationship with light levels
The relationship between nest densities (nests km−1 year−1) by species/subpopulation and the average light levels during 2008–2012 was analysed using a generalised additive model (GAM) and the function gamm (generalised additive mixed modelling) of the R-package nlme (Pinheiro et al. 2018). The response variable (nest density) was determined as a function of two continuous covariates: average light levels and latitude. Each ~ 1 km beach segment contributed to the distribution only once (n = 347 km for the northern loggerhead turtle stock, hawksbill and olive ridley turtles, n = 257 km for the southern loggerhead turtle stock, n = 159 km for leatherback turtles). Statistical analyses were carried out using the software R 3.5.1 (R Core Team 2018) and we used a significance level of α = 0.05.
Reproductive hotspots
Based on nest densities (nests km−1 year−1) during the most recent period (2008–2012), we identified two types of reproductive hotspots for each species/subpopulation: (1) nesting areas with very high- or high-nest density and high exposure to artificial light (> 5.5 DN) and (2) nesting areas with very high- or high-nest density and low exposure to artificial light. Those areas were grouped into beaches with variable extents (2 to 37 km), according to standard methodology adopted by TAMAR. A given beach was considered potentially exposed or to have had observed changes in brightness when more than 50% of the hotspot segments were located in areas potentially exposed to light pollution (> 5.5 DN) or had observed changes in brightness (> 3 DN, Table 1). We used TAMAR’s dataset (2008–2012) to classify these beaches according to (1) the type of management strategy used: clutches could be either left in situ, relocated to another section of the beach or, on a lesser extent, to open-air hatcheries. Nest relocations are required in some areas to protect clutches from beach erosion, risk of flooding, fox predation or illegal egg take by humans, or other anthropogenic impacts such as light induced misorientation, in places where there is extensive coastal development (Marcovaldi et al. 2005); (2) the existence of documented cases of hatchling orientation problems (a disruption in the ability of hatchlings to detect or to respond to the cues needed for an oriented crawl towards the sea; Lorne and Salmon 2007), identified during beach patrols through examination of hatchling tracks. The hotspot sites were classified regarding the existence of recorded hatchling orientation problems according to the scores: 0 = no cases or 1 = recorded cases of hatchling orientation problems. The proportion of sites with hatchling orientation problems was calculated considering the sites that had recorded orientation problems divided by the total number of sites and (3) if the presence of artificial light influences the management strategy used locally, defined as whether clutches on a given beach or section of a beach needed to be relocated because of the presence of light or not.
Results
All species/subpopulations showed an increase in the proportion of nesting areas potentially exposed to light pollution (> 5.5 DN) between the periods (1992–1996 and 2008–2012). The overall proportion of nesting areas potentially exposed increased from 34.1% during the first period (1992–1996) to 61.1% during the most recent period (2008–2012; Fig. 2 and Table 2). Overall, increase in brightness (> 3 DN) was experienced in 63.7% of the area. None of the nesting areas experienced a decrease in brightness (> 3 DN). Considering the annual nesting numbers during the most recent period (2008–2012), a variable proportion of nests for the different species/subpopulations were located in areas potentially exposed to light pollution (Table 2; 28.7–80.5%).
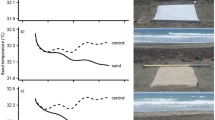
Change in brightness seen across nesting areas in Brazil. A digital number (DN) between 0 and 63 represents each pixel. Zero represents darkness, while brightly lit areas saturate at values of 63. a Bahia and Sergipe, nesting grounds for the northern Caretta caretta stock (NCc), Eretmochelys imbricata (Ei) and Lepidochelys olivacea (Lo) turtles. b Espírito Santo, nesting grounds for the southern Caretta caretta stock (SCc) and Dermochelys coriacea (Dc) turtles, and c Rio de Janeiro, nesting grounds for the southern Caretta caretta stock (SCc)
We identified 253 ~ 1 km segments which had either very high- or high-nesting density for any of the species/subpopulations. Those reproductive hotspots were grouped into 54 sites, important for one or more marine turtle species (Table 1). Of those very high- and high-nesting density sites, 37.1% were located in areas with no/low exposure to light, the remaining 62.9% were in areas potentially exposed to light pollution and 64.8% of hotspots had experienced increasing light levels. The proportion of reproductive hotspots potentially exposed to light pollution varied among each species/subpopulation, ranging from 26.7% in olive ridley turtles to 75.9% in hawksbill turtles (Table 3). In 55% of reproductive hotspots there are documented cases of hatchling orientation problems (classified either as ‘misorientation’, when the hatchlings crawl towards the lights; or ‘disorientation’, when hatchlings are incapable of crawling in any specific direction; Verheijen 1985) and in 42.6% the presence of artificial light influences local management strategies, however in most cases it means a small proportion of nests located in specific areas, rather than those across an entire beach or whole ~ 1 km segment (n = 54; Table 1). The proportion of reproductive hotspots potentially exposed to light pollution also varied considering the different states, being 46.1% in Sergipe, 60.7% in Bahia, 50% in Espírito Santo and 100% in Rio de Janeiro.
The GAM models suggested that light had a significant effect on nest densities of hawksbills and the northern loggerhead turtle stock (approximate significance of the smooth term: estimated degrees of freedom = 1.05, F = 11.58, p = 0.0001 and estimated degrees of freedom = 5.39, F = 15.34, p < 0.0001, respectively, Fig. 3). Latitude had a significant effect on nest densities of all species (olive ridley turtles estimated degrees of freedom = 7.7, F = 18.30, p < 0.0001; leatherback turtles estimated degrees of freedom = 6.51, F = 38.98, p < 0.0001; southern loggerhead turtle stock estimated degrees of freedom = 5.31, F = 18.47, p < 0.0001, northern loggerhead turtle stock estimated degrees of freedom = 2.88, F = 29.49, p < 0.0001, hawksbill turtles estimated degrees of freedom = 8.38, F = 25.70, p < 0.0001; Fig. 3).
Graphical summary of GAMM model fits for nesting densities. Covariates are shown on x-axis: average light levels in DN (left side) and latitude (right side) for a, b olive ridley turtle (n = 347), c, d leatherback turtle (n = 159), e, f hawksbill turtle (n = 347), g, h northern loggerhead turtle stock (n = 347) and i, j southern loggerhead turtle stock (n = 257). Note different x and y-axis scales. In j, the region between the two vertical lines represent latitudes where no nesting data was collected
Discussion
The majority of turtle nesting areas analysed here experienced increases in brightness between 1992–1996 and 2008–2012 (Fig. 2), suggesting the increasing trend in artificial lighting has the potential to represent a significant anthropogenic impact for coastal habitats and marine turtles in Brazil. The human population in Brazil has grown considerably throughout the study period and much of the population growth was seen in coastal areas, where the largest urban centres are currently located (Instituto Brasileiro de Geografia e Estatística 2013). With an extensive and diverse coastline, coastal development and artificial lighting have evolved distinctly among regions in Brazil, mostly driven by tourism (Lopez et al. 2015), industrial activities or the development of the communities themselves.
Long-term conservation efforts started by TAMAR in the 1980s is thought to have contributed to recent increases in nesting numbers for all four mainly continentally nesting species, indicating signs of population recovery (Baptistotte et al. 2003; da Silva et al. 2007; Marcovaldi et al. 2007; Marcovaldi and Chaloupka 2007; Colman et al. 2019). This is in parallel with many other marine turtle populations globally (Mazaris et al. 2017). In Brazil, the estimated annual population growth rate is variable among species/subpopulations, ranging from 4 to 12% per year (TAMAR, unpublished data). The fact, however, that both variables (annual nesting numbers and average light levels) are increasing suggests that if light is managed well, turtles and humans can co-exist in Brazil. As coastal development continues to progress, future conflicts could arise, and continued management of artificial light will be key. In northern Bahia, high marine turtle nesting densities are observed where relatively high average light levels were recorded by the satellite images. Identifying the impact of one disturbance when a population is recovering from the alleviation of others is a challenging task.
Other studies have highlighted the importance of detecting temporal changes in artificial light exposure of marine turtle nesting areas (Kamrowski et al. 2012, 2014). Additionally, here we identified hotspots (Table 1), areas with high reproductive importance, which may either be impacted by artificial light, thereby representing zones requiring management attention (since light levels have been increasing in Brazil), or dark areas which are likely candidates for future and continued protection (Fuentes et al. 2016). The majority of the reproductive hotspots identified here are located in areas considered potentially exposed to artificial light, however, those areas are generally bright as a result of skyglow, which is the scattering of upwardly reflected artificial light in the atmosphere and reflection by clouds (Davies et al. 2014). Specific legislation prohibits direct light incidence on nesting beaches (IBAMA normative no. 11, from 31 January 1995) in Brazil. However, across globally important nesting areas for hawksbill and loggerhead turtles such as northern Bahia (Marcovaldi and Chaloupka 2007; Marcovaldi et al. 2007) and Rio de Janeiro coasts (Lima et al. 2012), relocation is still used as a strategy for managing nests in lit areas. Nest relocation can, however affect hatchling sex ratios (Godfrey and Mrosovsky 1999) and could cause long-term genetic consequences (Mrosovsky 2006).
Light levels and nesting density had variable relationships across the species/subpopulations, reflecting the interactions between nest site selection and the variable patterns of artificial light along the Brazilian coast. Olive ridley and leatherback turtles have their core rookeries within long established dark and protected areas—Santa Isabel and Comboios Biological Reserves, established in 1988 and 1984, respectively. Those species are still of conservation concern, however, as coastal development continues to pressure the surroundings of the protected areas. An increase in nesting numbers caused by population recovery could also result in broader geographical distributions, as turtles could re-colonise previous nesting areas, creating conflicts with humans.
For hawksbills and the northern loggerhead stock, the relationship between nest density and average light levels at the individual beach scale varied, with high nesting density also seen in areas where there were relatively high average light levels (Fig. 3e, g). This suggests that other drivers for nest site selection, such as temperature gradients, beach and horizon elevation and sand moisture (Wood and Bjorndal 2000; Pendoley and Kamrowski 2015), may play a more important role than the presence/absence of artificial light. For the hatchlings, to emerge in areas with relatively high average light levels, the lunar phase can be an important factor influencing orientation, and also site-specific topographic features such as vegetation, sand dunes and beach slope, which can help hatchlings in finding their way to the ocean. Another possibility is that turtles may not be exposed to as much light as suggested by the satellite data, i.e. it may be due to the coarse scale of the light data not allowing for differences at the local beach scale to be considered. Loggerhead, green and leatherback turtle nest densities, however, have been suggested as being negatively influenced by artificial light levels at the individual beach scale at other nesting sites such as Florida (Weishampel et al. 2016; Hu et al. 2018) and eastern Mediterranean (Mazor et al. 2013). In Brazil, in heavily industrialised coastal areas such as Rio de Janeiro state, all reproductive hotspots identified are located in areas potentially exposed to light pollution (Table 1). The existence of high nesting densities in lit areas suggests turtles can tolerate quite high levels of disturbance (Kamrowski et al. 2012). Further research should investigate the potential interaction and cumulative effects of other factors affecting the response variable, in this case nest density, operating in finer scale (e.g. species-specific nest site selection, physical characteristics of the beaches and spatial autocorrelation of the nesting data) in comparison to artificial light.
Despite being of great utility to marine turtle management and conservation in Brazil, the interpretation of our findings requires a number of considerations of the spatial, temporal and spectral resolution limitations of the DMSP OLS sensors data as a proxy to estimate the effects of light pollution on marine turtles. Firstly, the 1 × 1 km pixel scale is much coarser than the width of most Brazilian beaches, including brightness that can be inland. Secondly, the measure of light as viewed from the sky might not represent the light as perceived by turtles from the beach. On-ground assessments of the impacts of light pollution are needed to confirm the identified levels of exposure (Kamrowski et al. 2014) and to establish thresholds of exposure at which light affects individual turtles and their populations (Fuentes et al. 2016; Lara et al. 2016). However, conducting those on-ground assessments are logistically challenging, especially when considering pan-national scales such as the present study (604 km across four different states in Brazil). A study conducted in Rio de Janeiro measured on-ground light intensity using light meters, and highlights the challenges of measuring artificial light, with results suggesting that hatchlings had orientation problems evens when the light meters read ‘0 lx’, which is the measure adopted by the Brazilian legislation (Lara et al. 2016).
Thirdly, the Stable Light DMSP product represents an annual average, including periods outside the nesting season. Beach use increases during the Austral summer months, coinciding with the peak of the nesting season (December and January). If the resolution of the data were finer, those seasonal changes could be captured more precisely. A newer product with finer spatial resolution, the Visible Infrared Imaging Radiometer Suite (VIIRS), has been introduced as a successor to DMSP-OLS in 2012, and used to assess impacts of light pollution and coastal development on sea turtles in Florida (Fuentes et al. 2016; Hu et al. 2018). Future assessments of temporal changes in light adjacent to marine turtle nesting areas could use these finer-resolution monthly nightlight data (Kamrowski et al. 2014), even though the archive only starts in 2012 (Miller et al. 2013), currently preventing the assessment of long-term changes. However, we recognise that VIIRS also has some limitations, such as not detecting short wavelengths, which are hatchlings usually most sensitive to, and includes lights from flares and fires, potentially giving a false indication of light. Finally, in this study we did not use habitat or landscape features other than light and nesting locations. Consequently, some important explanatory variables might have not been considered, and there is the possibility that light covaries with measures that we are not yet accounting for. This would be worthy of further investigation.
Coastal artificial light constitutes a potential threat for marine turtles, however offshore lighting, such as from oil rigs, is known to attract fish of commercial interest (Marchesan et al. 2005). Research has shown that marine turtle hatchlings could be attracted to coastal artificial lights while in nearshore waters, increasing predation risk (Thums et al. 2016) and having potential impacts on population recruitment. There is a need for regional assessments to evaluate the impacts of offshore and near shore lighting (such as light from ports, jetties and moored vessels waiting to enter ports close to rookeries; Wilson et al. 2018) on marine turtle hatchlings dispersing from natal beaches in Brazil. Their dispersion routes are very likely to overlap with productive oil and gas fields, located near Sergipe, Espírito Santo and Rio de Janeiro coasts (Fig. 2b–e), representing a potential ecological sink for hatchlings.
Light pollution can affect marine turtles and their habitats and quantifying the impacts at a population-level remains a challenge to ecologists (Davies and Smyth 2018). If lighting is unsuitable during the hatchling emergence period, hatchlings could have direct fitness consequences, such as reduced energy during their frenzy period offshore, which appears essential for reaching the “favourable currents” that facilitate reaching nursery habitats (Scott et al. 2017). A study conducted in Mediterranean nesting sites estimated that nightlight could result in an additional reduction of recruitment of up to 6% (Dimitriadis et al. 2018). For species with late maturity such as marine turtles, with relative uncertainty regarding typical time to reach sexual maturity, the impact on population recruitment could take many decades to fully manifest in nesting numbers.
Human-wildlife conflicts where coastal development overlaps with nesting areas can be effectively managed with the establishment of dark and protected areas, the existence and enforcement of specific legislation or ultimately with nest relocation. The reproductive hotspots identified here can be used as guidance in future management decisions considering marine turtles in Brazil, identifying areas where intervention is needed and those candidates for continued protection. Perhaps most notable is the fact that conservation strategies used by TAMAR in Brazil during the last 35 years have heavily relied on the involvement of local communities, with the development of varied environmental awareness activities, adapted to the socio-environmental evolving contexts of the different locations (da Silva et al. 2016). Evaluating the effects of anthropogenic factors on sea turtle habitats was one of the 20 questions pointed out as research priorities for marine turtles (Hamann et al. 2010), and was considered still insufficiently addressed in a recent review of the peer-reviewed literature (Rees et al. 2016). As coastal development increases, not only in Brazil, but worldwide, the use of satellite imagery is still limited in application and scope, however it has potential and with technological advances it should improve to be a valuable tool to monitor medium to long-term trends in light and to evaluate potential impacts of light on marine turtles and their habitats.
References
Almeida AP, Moreira LMP, Bruno SC, Thomé JCA, Martins AS, Bolten AB, Bjorndal KA (2011) Green turtle nesting on Trindade Island, Brazil: abundance, trends, and biometrics. Endanger Species Res 14:193–201
Aubrecht C, Elvidge CD, Longcore T, Rich C, Safran J, Strong AE, Eakin CM, Baugh KE, Tuttle BT, Howard AT, Erwin EH (2008) A global inventory of coral reef stressors based on satellite observed nighttime lights. Geocarto Int 23:467–479
Baptistotte C, Thomé JC, Bjorndal KA (2003) Reproductive biology and conservation status of the loggerhead sea turtle (Caretta caretta) in Espírito Santo State, Brazil. Chelonian Conserv Biol 4:1–7
Bellini C, Santos AJB, Grossman A, Marcovaldi MA, Barata PCR (2013) Green turtle (Chelonia mydas) nesting on Atol das Rocas, north-eastern Brazil, 1990-2008. J Mar Biol Assoc UK 93:1117–1132
Bennie J, Duffy JP, Davies TW, Correa-Cano ME, Gaston KJ (2015) Global trends in exposure to light pollution in natural terrestrial ecosystems. Remote Sens 7:2715–2730
Cinzano P, Falchi F, Elvidge CD (2001) The first world atlas of the artificial night sky brightness. Mon Not R Astron Soc 328:689–707
Colman LP, Thomé JCA, Almeida AP, Baptistotte C, Barata PCR, Broderick AC, Ribeiro FA, Vila-Verde L, Godley BJ (2019) Thirty years of leatherback turtle (Dermochelys coriacea) nesting in Espírito Santo, Brazil, 1988-2017: reproductive biology and conservation. Endanger Species Res 39:147–158
Da Silva ACCD, De Castilhos JC, Lopez GG, Barata PCR (2007) Nesting biology and conservation of the olive ridley sea turtle (Lepidochelys olivacea) in Brazil, 1991/1992 to 2002/2003. J Mar Biol Assoc UK 87:1047–1056
Da Silva VRF, Mitraud SF, Ferraz MLCP, Lima EHSM, Melo MTD, Santos AJB, Silva ACC, Castilhos JC, Batista JAF, Lopez GG, Tognin F, Thomé JC, Baptistotte C, Silva BMG, Becker JH (2016) Adaptive threat management framework: integrating people and turtles. Environ Dev Sustain 18:1541–1558
Davies TW, Smyth T (2018) Why artificial light at night should be a focus for global change research in the 21st century. Glob Change Biol 24:872–882
Davies TW, Duffy JP, Bennie J, Gaston KJ (2014) The nature, extent, and ecological implications of marine light pollution. Front Ecol Environ 12:347–355
Dimitriadis C, Fournari-Konstantinidou I, Sourbèsa L, Koutsoubas D, Mazaris AD (2018) Reduction of sea turtle population recruitment caused by nightlight: evidence from the Mediterranean region. Ocean Coast Manage 153:108–115
Duffy JP, Bennie J, Durán AP, Gaston KJ (2015) Mammalian ranges are experiencing erosion of natural darkness. Sci Rep 5:12042
Elvidge CD, Imhoff ML, Baugh KE, Hobson VR, Nelson I, Safran J, Dietz JB, Tuttle BT (2001) Night-time lights of the world: 1994-1995. ISPRS J Photogramm Remote Sens 56:81–99
Elvidge CD, Cinzano P, Pettit DR, Arvesen J, Sutton P, Small C, Nemani R, Longcore T, Rich C, Safran J, Weeks J, Ebener S (2007) The Nightsat mission concept. Int J Remote Sens 28:2645–2670
Freitas JRd, Bennie J, Mantovani W, Gaston KJ (2017) Exposure of tropical ecosystems to artificial light at night: Brazil as a case study. PLoS ONE 12(2):e0171655. https://doi.org/10.1371/journal.pone.0171655
Fuentes MMPB, Gredzens C, Bateman BL, Boettcher R, Ceriani SA, Godfrey MH, Helmers D, Ingram DK, Kamrowski RL, Pate M, Pressey RL, Radeloff VC (2016) Conservation hotspots for marine turtle nesting in the United States based on coastal development. Ecol Appl 26:2706–2717
Godfrey MH, Mrosovsky N (1999) Estimating hatchling sex ratios. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4. pp 136–138.
Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA, Bolten AB, Broderick AC, Campbell LM, Carreras C, Casale P, Chaloupka M, Chan SKF, Coyne MS, Crowder LB, Diez CE, Dutton PH, Epperly SP, FitzSimmons NN, Formia A, Girondot M, Hays GC, Cheng IJ, Kaska Y, Lewison R, Mortimer JA, Nichols WJ, Reina RD, Shanker K, Spotila JR, Tomás J, Wallace BP, Work TM, Zbinden J, Godley BJ (2010) Global research priorities for sea turtles: Informing management and conservation in the 21st century. Endanger Species Res 11:245–269
Hu Z, Hu H, Huang Y (2018) Association between nighttime artificial light pollution and sea turtle nest density along Florida coast: a geospatial study using VIIRS remote sensing data. Environ Pollut 239:30–42
Instituto Brasileiro de Geografia e Estatística (2013) Atlas do Censo Demográfico 2010. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro
Kamrowski R, Limpus C, Moloney J, Hamann M (2012) Coastal light pollution and marine turtles: assessing the magnitude of the problem. Endanger Species Res 19:85–98
Kamrowski RL, Limpus C, Jones R, Anderson S, Hamann M (2014) Temporal changes in artificial light exposure of marine turtle nesting areas. Glob Change Biol 20:2437–2449
Lara PH, Almeida DT, Famigliettia C, Romano A, Whelpley J, Byun A (2016) Continued light interference on loggerhead hatchlings along the Southern Brazilian Coast. Mar Turt Newls 149:1–5
Levenson DH, Eckert SA, Crognale MA, Deegan JF, Jacobs GH (2004) Photopic spectral sensitivity of green and loggerhead sea turtles. Copeia 4:908–914
Lima EPE, Wanderlinde J, Almeida DT, Lopez G, Goldberg DW (2012) Nesting ecology and conservation of the loggerhead sea turtle (Caretta caretta) in Rio de Janeiro, Brazil. Chelonian Conserv Biol 11:249–254
Longcore T, Rich C (2004) Ecological light pollution. Front Ecol Environ 2:191–198
Lopez GG, Saliés EC, Lara PH, Tognin F, Marcovaldi MA, Serafini TZ (2015) Coastal development at sea turtles nesting ground: efforts to establish a tool for supporting conservation and coastal management in northeastern Brazil. Ocean Coast Manage 116:270–276
Lorne J, Salmon M (2007) Effects of exposure to artificial lighting on orientation of hatchling sea turtles on the beach and in the ocean. Endanger Species Res 3:23–30
Marchesan M, Spoto M, Verginella L, Ferrero EA (2005) Behavioural effects of artificial light on fish species of commercial interest. Fish Res 73:171–185
Marcovaldi MÂ, Chaloupka M (2007) Conservation status of the loggerhead sea turtle in Brazil: an encouraging outlook. Endanger Species Res 3:133–143
Marcovaldi MÂ, Marcovaldi GGD (1999) Marine turtles of Brazil: the history and structure of Projeto TAMAR-IBAMA. Biol Conserv 91:35–41
Marcovaldi MÂ, Patiri V, Thomé JC (2005) Projeto TAMAR-IBAMA: twenty-five years protecting Brazilian sea turtles through a community-based conservation programme. MAST 4:39–62
Marcovaldi MÂ, Lopez GG, Soares LS, Santos AJB, Bellini C, Barata PCR (2007) Fifteen years of hawksbill sea turtle (Eretmochelys imbricata) nesting in northern Brazil. Chelonian Conserv Biol 6:223–228
Marcovaldi MÂ, López-Mendilaharsu M, Santos AS, Lopez GG, Godfrey MH, Tognin F, Baptistotte C, Thomé JC, Dias ACC, Castilhos JC, Fuentes MMBP (2016) Identification of loggerhead male producing beaches in the south Atlantic: implications for conservation. J Exp Mar Biol Ecol 477:14–22
Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC (2017) Global conservation successes. Sci Adv 3:e1600730
Mazor T, Levin N, Possingham HP, Levy Y, Rocchini D, Richardson AJ, Kark S (2013) Can satellite-based night lights be used for conservation? The case of nesting sea turtles in the Mediterranean. Biol Conserv 159:63–72
Miller SD, Straka W, Mills SP, Elvidge CD, Lee TF, Solbrig J, Walther A, Heidinger AK, Weiss SC (2013) Illuminating the capabilities of the Suomi National Polar-Orbiting Partnership (NPP) Visible Infrared Imaging Radiometer Suite (VIIRS) Day/Night Band. Remote Sens 5:6717–6766
Mrosovsky N (2006) Distorting gene pools by conservation: assessing the case of doomed turtle eggs. Environ Manage 38:523–531
Okuyama J, Kitagawa T, Zenimoto K, Kimura S, Arai N, Sasai Y, Sasaki H (2011) Trans-Pacific dispersal of loggerhead turtle hatchlings inferred from numerical simulation modeling. Mar Biol 158:2055–2063
Pendoley K, Kamrowski RL (2015) Influence of horizon elevation on the sea-finding behaviour of hatchling flatback turtles exposed to artificial light glow. Mar Ecol Prog Ser 529:279–288
Pike DA (2013) Climate influences the global distribution of sea turtle nesting. Glob Ecol Biogeogr 22:555–566
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: linear and nonlinear mixed effects models. R package version 3.1-137. https://CRAN.R-project.org/package=nlme
Price JT, Drye B, Domangue RJ, Paladino FV (2018) Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol Conserv Biol 13:415–422
Putman NF (2018) Marine migrations. Curr Biol 28:952–1008
Putman NF, Bane JM, Lohmann KJ (2010a) Sea turtle nesting distributions and oceanographic constraints on hatchling migration. Proc Royal Soc B 277:3631–3637
Putman NF, Shay TJ, Lohmann KJ (2010b) Is the geographic distribution of nesting in the kemp’s ridley turtle shaped by the migratory needs of offspring? Integr Comp Biol 50:305–314
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rees A, Alfaro-Shigueto J, Barata P, Bjorndal K, Bolten A, Bourjea J, Broderick AC, Campbell LM, Cardona L, Carreras C, Casale P, Ceriani SA, Dutton PH, Eguchi T, Formia A, Fuentes MMBP, Fuller WJ, Girondot M, Godfrey MH, Hamann M, Hart KM, Hays GC, Hochscheid S, Kaska Y, Jensen MP, Mangel JC, Mortimer JA, Naro-Maciel E, Ng CKY, Nichols WJ, Phillott AD, Reina RD, Revuelta O, Schofield G, Seminoff JA, Shanker K, Tomás J, van de Merwe JP, Van Houtan KS, Vander Zanden HB, Wallace BP, Wedemeyer-Strombel KR, Work TM, Godley BJ (2016) Are we working towards global research priorities for management and conservation of sea turtles? Endanger Species Res 31:337–382
Reis EC, Soares LS, Vargas SM, Santos FR, Young RJ, Bjorndal KA, Bolten AB, Lôbo-Hadju G (2010) Genetic composition, population structure and phylogeography of the loggerhead sea turtle: Colonization hypothesis for the Brazilian rookeries. Conserv Genet 11:1467–1477
Salmon M (2003) Artificial lighting and sea turtles. The Biologist 50:163–168
Scott R, Biastoch A, Agamboue PD, Bayer T, Boussamba FL, Formia A, Godley BJ, Mabert BDK, Manfoumbi JC, Schwarzkopf FU, Sounguet GP, Wagner P, Witt MJ (2017) Spatio-temporal variation in ocean current-driven hatchling dispersion: Implications for the world’s largest leatherback sea turtle nesting region. Divers Distrib 23:604–614
Shillinger GL, Di Lorenzo E, Luo H, Bograd SJ, Hazen EL, Bailey H, Spotila JR (2012) On the dispersal of leatherback turtle hatchlings from Mesoamerican nesting beaches. Proc R Soc B 279:2391–2395. https://doi.org/10.1098/rspb.2011.2348
Silva E, Marco A, Da Graça J, Pérez H, Abella E, Patino-Martinez J, Martins S, Almeida C (2017) Light pollution affects nesting behavior of loggerhead turtles and predation risk of nests and hatchlings. J Photochem Photobiol B 173:240–249
Thums M, Whiting SD, Reisser J, Pendoley KL, Pattiaratchi CB, Proietti M, Hetzel Y, Fisher R, Meekan MG (2016) Artificial light on water attracts turtle hatchlings during their near shore transit. R Soc Open Sci 3:160142
Verheijen FJ (1985) Photopollution: artificial light optic spatial control systems fail to cope with. Incidents, causation, remedies. Exp Biol 44:1–18
Weishampel ZA, Cheng W-H, Weishampel JF (2016) Sea turtle nesting patterns in Florida vis-à-vis satellite-derived measures of artificial lighting. Remote Sens Ecol Conserv 2:59–72
Wilson P, Thums M, Pattiaratchi C, Meekan M, Pendoley K, Fisher R, Whiting S (2018) Artificial light disrupts the nearshore dispersal of neonate flatback turtles Natator depressus. Mar Ecol Prog Ser 600:179–192
Witherington BE, Bjorndal KA (1991) Influences of wavelength and intensity on hatchling sea turtle phototaxis: implications for sea-finding behavior. Copeia 4:1060–1069
Witherington BE, Martin RE (2000) Understanding, assessing, and resolving light-pollution problems on sea turtle nesting beaches. In: Florida Fish and Wildlife Conservation Commission, Marine Research Institute, St. Petersburg, FL, 84pp
Wood DW, Bjorndal KA (2000) Relation of temperature, moisture, salinity, and slope to nest site selection in loggerhead sea turtles. Copeia 1:119–128
Acknowledgements
We thank all staff and numerous other people involved in data collection over the several nesting seasons. LC acknowledges the support of a Science Without Borders scholarship from the National Council for Scientific and Technological Development (CNPq), Brazil, the University of Exeter, UK, and an additional grant from the Marine Turtle Conservation Fund, by the U.S. Fish and Wildlife Service. Fieldwork in Brazil was carried out by Fundação Pró-TAMAR, which is among the institutions responsible for implementing conservation actions under the National Action Plan for the Conservation of Sea Turtles in Brazil—NAP ICMBio/MMA, under the permit # 42760-11 from SISBIO (Authorization and Information System on Biodiversity), the research authorization system of ICMBio, Brazilian Ministry of Environment (MMA). JB acknowledges the support of Natural Environment Research Council (NERC; grant no: NE/P01156X/1), UK. The manuscript benefited from the detailed input of 3 referees and the Editor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: “Coastal and marine biodiversity”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colman, L.P., Lara, P.H., Bennie, J. et al. Assessing coastal artificial light and potential exposure of wildlife at a national scale: the case of marine turtles in Brazil. Biodivers Conserv 29, 1135–1152 (2020). https://doi.org/10.1007/s10531-019-01928-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-019-01928-z