Abstract
Backgrounds
Circadian rhythms are patterns of behaviour, physiology, and metabolism that occur within a period of approximately 24 h. The higher risk of breast and prostate cancers among shift workers, as well as the general population, are reported to be associated with circadian rhythm disruption caused by exposure to light at night. We focused on the effects of bright light before bed comparing effects between men and women.
Methods
Male and female healthy volunteers aged 20–30 were exposed to 4 hours of bright light before bed for 3 and 4 days.
Results
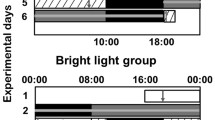
We analyzed the shift of circadian rhythms of subjects based on cortisol secretion patterns in response to short periods of bright-light exposure at bedtime. We also found an increase of oxidative stress including MDA, 8-OHdG, and total antioxidants in both male and female volunteers.
Conclusion
These results suggest that bright light exposure before sleep, often encounter in modern daily life, has a considerable influence on the human body. The chronic effects of light exposure before bed time such as the carcinogenic effects caused by circadian disruption and oxidative stress need further investigation.
Similar content being viewed by others
References
DiTacchio, L., DiTacchio, K. A. & Panda, S. Relevance of circadian rhythm in cancer. Energy Balance and Cancer 1–19 (2015).
Albers, S. & Duriscoe, D. Modeling light pollution from population data and implications for National Park Service lands. George Wright Forum 18, 56–68 (2001).
Cinzano, P., Falchi, F. & Elvidge, C. D. The first world atlas of the artificial night sky brightness. Mon Notices Royal Astron Soc 328, 689–707 (2001).
Stevens, R. G. et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med 2009, 053512 (2010).
Megdal, S. P., Kroenke, C. H., Laden, F., Pukkala, E. & Schernhammer, E. S. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer 41, 2023–2032 (2005).
Conlon, M., Lightfoot, N. & Kreiger, N. Rotating shift work and risk of prostate cancer. Environ Epidemiol 18, 182–183 (2007).
Schernhammer, E. S. et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst 95, 825–828 (2003).
Viswanathan, A. N., Hankinson, S. E. & Schernhammer, E. S. Night shift work and the risk of endometrial cancer. Cancer Res 67, 10618–10622 (2007).
Pauley, S. M. Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med Hypotheses 63, 588–596 (2004).
Rybnikova, N. A., Haim, A. & Portnov, B. A. Is prostate cancer incidence worldwide linked to artificial light at night exposures? Review of earlier findings and analysis of current trends. Arch Environ Occup Health 72, 111–122 (2017).
Kloog, I., Haim, A., Stevens, R. G., Barchana, M. & Portnov, B. A. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol Int 25, 65–81 (2008).
Stevens, R. G. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol 38, 963–970 (2009).
Kim, Y. J., Lee, E., Lee, H. S., Kim, M. & Park, M. S. High prevalence of breast cancer in light polluted areas in urban and rural regions of South Korea: An ecologic study on the treatment prevalence of female cancers based on National Health Insurance data. Chronobiol Int 32, 657–667 (2015).
Stevens, R. G. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology 16, 254–258 (2005).
Savvidis, C. & Koutsilieris, M. Circadian rhythm disruption in cancer biology. Mol Med 18, 1249–1260 (2012).
Ashkenazi, L. & Haim, A. Light interference as a possible stressor altering HSP70 and its gene expression levels in brain and hepatic tissues of golden spiny mice. J Exp Biol 215, 4034–4040 (2012).
Arendt, J. Melatonin, circadian rhythms, and sleep. N Engl J Med 343, 1114–1116 (2000).
Corbella, O. & Yannas, S. Environmental study of two shopping malls in Rio de Janeiro. Eco-friendly 98, 483–486 (2014).
Cho, C.-H. et al. Molecular circadian rhythm shift due to bright light exposure before bedtime is related to subthreshold bipolarity. Sci Rep 6, 31846 (2016).
Schwartzbaum, J., Ahlbom, A. & Feychting, M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health 33, 336–343 (2007).
Kim, K. Y., Lee, E., Kim, Y. J. & Kim, J. The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea. Chronobio lInt 1–9 (2016).
Kim, Y. J., Park, M. S., Lee, E. & Choi, J. W. High incidence of breast cancer in light-polluted areas with spatial effects in Korea. Asian Pac J Cancer Prev 17, 361–367 (2016).
Koller, M. et al. Different patterns of light exposure in relation to melatonin and cortisol rhythms and sleep of night workers. J Pineal Res 16, 127–135 (1994).
Fonken, L. K. & Nelson, R. J. Illuminating the deleterious effects of light at night. F1000 Med Rep 3, 18 (2011).
Kyba, C. C. et al. Citizen science provides valuable data for monitoring global night sky luminance. Sci Rep 3, 1835 (2013).
Navara, K. J. et al. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43, 215–224 (2007).
Davis, S., Mirick, D. K. & Stevens, R. G. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93, 1557–1562 (2001).
Fonken, L. K. et al. Light at night increases body mass by shifting the time of food intake. PNAS 107, 18664–18669 (2010).
Shouse, J. Light of night. Educational Forum, Taylor & Francis 10, 150–150 (1946).
Krieger, D. T., Allen, W., Rizzo, F. & Krieger, H. P. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab 32, 266–284 (1971).
Hellhammer, D. H., Wüst, S. & Kudielka, B. M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34, 163–171 (2009).
Santiago, L. B., Jorge, S. M. & Moreira, A. C. Longitudinal evaluation of the development of salivary cortisol circadian rhythm in infancy. J Clin Endocrinol Metab 44, 157–161 (1996).
Ruis, M. A. et al. The circadian rhythm of salivary cortisol in growing pigs: effects of age, gender, and stress. Physiol Behav 62, 623–630 (1997).
Schulz, P., Kirschbaum, C., Prüßner, J. & Hellhammer, D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Health 14, 91–97 (1998).
Wolf, O. T., Schommer, N. C., Hellhammer, D. H., McEwen, B. S. & Kirschbaum, C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 26, 711–720 (2001).
Vahter, M., Åkesson, A., Lidén, C., Ceccatelli, S. & Berglund, M. Gender differences in the disposition and toxicity of metals. Environ Res 104, 85–95 (2007).
Kiyohara, C. & Ohno, Y. Sex differences in lung cancer susceptibility: a review. Gender Med 7, 381–401 (2010).
Dorak, M. T. & Karpuzoglu, E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet 3, 268 (2012).
Hardeland, R. & Burkhardt, S. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20, 921–962 (2003).
Coto-Montes, A. & Hardeland, R. Diurnal rhythm of protein carbonyl as an indicator of oxidative damage in Drosophila melanogaster: Influence of clock gene alleles and deficiencies in the formation of free-radical scavengers. Biol Rhythm Res 30, 383–391 (1999).
Hardeland, R., Coto-Montes, A. & Poeggeler, B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20, 921–962 (2003).
Kosugi, H., Enomoto, H., Ishizuka, Y. & Kikugawa, K. Variations in the level of urinary thiobarbituric acid reactant in healthy humans under different physiological conditions. Biol Pharm Bull 17, 1645–1650 (1994).
Coto-Montes, A. et al. Porphyric enzymes in hamster Harderian gland, a model of damage by porphyrins and their precursors. A chronobiological study on the role of sex differences. Chem-Biol Interact 134, 135–149 (2001).
Coto-Montes, A. et al. Physiological oxidative stress model: Syrian hamster Harderian gland-sex differences in antioxidant enzymes. Free Radic Biol Med 30, 785–792 (2001).
Burmistrov, S., Arutyunyan, A., Stepanov, M., Oparina, T. & Prokopenko, V. Effect of chronic inhalation of toluene and dioxane on activity of free radical processes in rat ovaries and brain. Bulletin Exp Biol Med 132, 832–836 (2001).
Dizdaroglu, M., Jaruga, P., Birincioglu, M. & Rodriguez, H. Free radical-induced damage to DNA: mechanisms and measurement 1, 2. Free Radic Biol Med 32, 1102–1115 (2002).
Gery, S. et al. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22, 375–382 (2006).
Winston, G. W. Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C Toxicol Pharmacol 100, 173–176 (1991).
Di Giulio, R. T., Washburn, P. C., Wenning, R. J., Winston, G. W. & Jewell, C. S. Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8, 1103–1123 (1989).
Foyer, C. H. & Noctor, G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17, 1866–1875 (2005).
Halliwell, B. Free radicals and antioxidants: updating a personal view. Nutr Rev 70, 257–265 (2012).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8, 579–591 (2009).
Yamato, M., Egashira, T. & Utsumi, H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic Biol Med 35, 1619–1631 (2003).
Borrás, C. et al. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34, 546–552 (2003).
Brown, M. K. & Naidoo, N. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol Neurobiol 42, 103–113 (2010).
Lungato, L. et al. Sleep deprivation alters gene expression and antioxidant enzyme activity in mice splenocytes. Scand J Immunol 77, 195–199 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HS., Lee, E., Moon, JH. et al. Circadian disruption and increase of oxidative stress in male and female volunteers after bright light exposure before bed time. Mol. Cell. Toxicol. 15, 221–229 (2019). https://doi.org/10.1007/s13273-019-0025-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-019-0025-9