Abstract
Objective
To observe the clinical effectiveness of Qinghuang Powder (青黄散, QHP) combined with Bupi Yishen Decoction (补脾益肾汤, BPYS) in treating myelodysplastic syndrome (MDS), and its relationship with France, America, and Britain (FAB) type, international prognosis scaling system (IPSS) risk, and chromosome karyotype.
Methods
There were 124 MDS patients subjected to the tests. By FAB typing, 91 patients were typed as refractory anemia (RA) type and 33 as refractory anemia with excess of blasts (RAEB) type; by IPSS scale, 21 were sorted to low risk, 77 to moderate risk I, 20 to moderate risk II, and 6 to high risk; 78 of them had normal chromosome and 46 with abnormal chromosome, including 26 of trisomy 8. All patients were treated with QHP+BPYS, and the changes of peripheral blood figure and bone marrow were observed.
Results
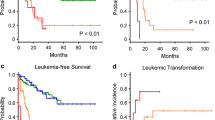
After treatment, the general effective rate was 72.58% (90/124), which in the patients of RA type was 80.22% (73/91) and in RAEB type 51.52% (17/33). The former was better than that in the later (P<0.01). For the analysis in the patients of different IPSS risk degrees, the effective rate was 95.24% (20/21) in the lowrisk group, 72.73% (56/77) in moderate risk I, 65.00% (13/20) in moderate-risk II, and 16.67% (1/6) in high-risk group. Those in the first two groups were superior to that in the latter two (P<0.01). The effective rate was 79.49% (61/78) in the patients with normal chromosome and was 60.87% (28/46) in the patients with abnormal chromosome, showing a significant difference between them. While in the patients of trisomy 8, it was 73.08% (19/26), which was parallel to that in the patients with normal chromosome.
Conclusion
The effectiveness of QHP+BPYS comprehensive therapy for MDS is unquestionably good, and it is markedly correlated with the FAB type and IPSS risk degree of the disease, as well as the normality of chromosome in the patient.
Similar content being viewed by others
References
Xu S, Hu XM, Xu YG. Effect of treatment for myelodysplastic syndrome by Qinghuang Powder combined with Chinese herbs for reinforcing Shen and strengthening Pi. Chin J Integr Tradit West Med (Chin) 2008;28:216–219.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982;51:189–199.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89:2079–2088.
Mitelman F. ISCN (1995). An international system for human cytogenetic nomenclature. Basel, Swizeland: S. Karger; 1995.
Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000;96:3671–3674.
William Blum. How much? How frequent? How long? A clinical guide to new therapies in myelodysplastic syndromes. Hematol Am Soc Hematol Educ Program 2010:314-321.
Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 2007;110:4385–4395.
Germing U, Gattemann N, Strupp C, Aivado M, Aul C. Validation of the WHO proposal for a new classifications of primary myelodysplastic syndromes: a retrospective analysis of 1 600 patients. Leukemia Res 2000;25:983–993.
Haase D, Fonatsch C, Freund M, Wörmann B, Bodenstein H, Bartels H, et al. Cytogenetic findings in 179 patients with myelodysplastic syndromes. Ann Hematol 1995;70:171–178.
Ma R, Hu XM. Treatment focused on myelodysplastic syndromes based on cytogenetic categories. J Leukemia Lymphoma (Chin) 2010;19:262–264.
Chen GQ, Zhu J, Shi X G, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic troxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with down regulation of Bcl-2 expression and modulation of PML-RARα/PML proteins. Blood 1996;88:1052.
Chen Z, Chen GQ, Shen ZX, Chen SJ, Wang ZY. Treatment of acute leukemia with arsenic compounds: in vitro and in vivo studies. Semin Hematol 2001;38:26.
Xu S. The clinical study of combinative treatment of Huayu Bushen method with androgen in myelodysplastic syndromes. Beijing: Doctoral Dissertation of China Academy of Chinese Medical Sciences; 2004.
Xu S, Xu YG, Yang XH, Hu XM, Wang HZ, Liu F, et al. Analysis of the quantity and function of innate immunologic cell in patients of myelodysplatic syndrome (MDS). Chin J Immun (Chin) 2009;25:596–600.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 30973903, 30973812)
Rights and permissions
About this article
Cite this article
Xu, S., Ma, R., Hu, Xm. et al. Clinical observation of the treatment of myelodysplastic syndrome mainly with Qinghuang Powder (青黄散). Chin. J. Integr. Med. 17, 834–839 (2011). https://doi.org/10.1007/s11655-011-0894-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-011-0894-9