Abstract
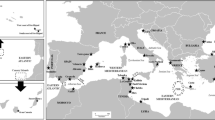
We studied the genetic structure of populations of the Atlanto-Mediterranean ascidian Clavelina lepadiformis (Müller, 1776). A 369 bp segment of the COI mitochondrial gene was sequenced in Mediterranean and Atlantic populations from inside harbours, marinas and fjords (interior populations), and from the open rocky littoral (exterior populations). Previous work identified genetic differences between C. lepadiformis inhabiting Mediterranean harbours and the Mediterranean rocky littoral, however, the origin of these two clades remained speculative. Here we compared the Mediterranean populations with four Atlantic populations (two interior and two exterior). Gene differentiation and maximum likelihood analyses showed that the Atlantic forms were not divided into interior and exterior clades, and were closely related to the interior clade in the Mediterranean. The results support the hypothesis that both clades evolved allopatrically in the two seas, and that a recent colonisation of Mediterranean marinas from the Atlantic was caused by ship-hull transport. Colonisation of habitats by new genetic variants, morphologically indistinguishable from local populations, may be common among benthic invertebrates, and only genetic tools can uncover these cryptic invasions.
Similar content being viewed by others
References
Baric, S. & C. Sturmbauer, 1999. Ecological parallelism and cryptic species in the genus Ophiothrix derived from mitochondrial DNA sequences. Mol. Phyl. Evolut. 11: 157–162.
Berrill, N. J., 1950. The tunicata. With an account of the British species. Ray Society, London. 354 pp.
Boisselier-Dubayle, M. C. & S. Gofas, 1999. Genetic relationships between marine and marginal marine populations of Cerithium species from the Mediterranean Sea. Mar. Biol. 135: 671–682.
Brunetti, R., 1979. Polyandrocarpa zorritensis (Van Name, 1931) a colonial ascidian new to the Mediterranean record. Vie Milieu 29: 647–652.
Carlton, J. T. & J. B. Geller, 1993. Ecological roulette: the global transport of nonindigenous marine organisms. Science 261: 78- 82.
Côrte-Real, H. B., J. P. Hawkins & J. P. Thorpe, 1996. Population differentiation and taxonomic status of the exploited limpet Patella candei in the Macaronesian islands (Azores, Madeira, Canaries). Mar. Biol. 125: 141–152.
Dalby, J. E., 1997. Dimorphism in the ascidian Pyura stolonifera near Melbourne, Australia, and its evaluation through field transplant experiments. PSZNI: Mar. Ecol. 18: 253–271.
De Caralt, S., S. López-Legentil, I. Tarjuelo, M. J. Uriz & X. Turon, 2002 Contrasting biological traits of Clavelina lepadiformis (Ascidiacea) populations from inside and outside harbours in the western Mediterranean. Mar. Ecol. Prog. Ser. 244: 125–137.
Felsenstein, J., 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17: 368–376.
Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791.
Harant, H., 1927. La faune Ascidiologique de Banyuls et Cette. Essai de revision des Ascidies de la Méditerranée occidentale. Annl. Inst. océanogr., Paris 4: 209–251.
Kimura, M., 1981. Estimation of evolutionary distances between homologous nucleotide sequences. Proc. natn. Acad. Sci. U.S.A. 78: 454–458.
Koukouras, A., E. Voultsiadou-Koukoura, T. Kevrekidis & D. Vafidis,1995. Ascidian fauna of the Aegean sea with a check list of eastern Mediterranean and Black sea species. Annl. Inst. océanogr., Paris 71: 19–34.
Lambert, C. C. & G. Lambert, 1998. Non-indigenous ascidians in southern California harbors and marinas. Mar. Biol. 130: 675- 688.
Latorre, A., A. Moya & F. Ayala, 1986. Mitochondrial DNA polymorphism of Drosophila subobscura. Proc. natn. Acad. Sci. U.S.A. 83: 8649–8653.
Lynch, M. & T. J. Crease, 1990. The analysis of population survey data on DNA sequence variation. Mol. Biol. Evol. 7: 377–394.
Maldonado, A., 1985. Evolution of the Mediterranean Basins and a detailed reconstruction of the Cenozoic paleoceanography. In Margalef, R. (ed.), Western Mediterranean. Pergamon Press, Oxford: 17–60.
McFadden, C. S., 1999. Genetic and taxonomic relationships among Northeastern Atlantic and Mediterranean populations of the soft coral Alcyonum coralloides. Mar. Biol. 133: 171–184.
Millar, R. H., 1966. Marine invertebrates of Scandinavia. 1. Tunicata Ascidiacea. Scandinavian University Books, Oslo. 123 pp.
Monniot, C., 1981. Apparition de l'ascidie Microcosmus exasperatus dans les ports méditerranéens. Tethys 10: 59–62.
Monniot, C. & F. Monniot, 1994. Additions to the inventory of eastern tropical atlantic ascidians: arrival of cosmopolitan species. Bull. mar. Sci. 54: 71–93.
Monniot, C., F. Monniot & P. Laboute, 1985. Ascidies du port de Papeete (Polynèsie française): relations avec la milieu naturel et apports intercontinentaux par la navigation. Bull. Mus. natn. Hist., Paris 7A: 481–495.
Monniot, C., F. Monniot F & P. Laboute, 1991. Coral Reef Ascidians of New Caledonia. O.R.S.T.O.M., Paris. 247 pp.
Nei, M., 1982. Evolution of human races at the gene level. In Bonne-Tamir, B., T. Cohen & R. M. Goodman (eds), Human Genetics, Part A. The Unfolding Genome. Alan R. Liss, New York: 167- 181.
Nei, M.,1987. Molecular Evolutionary Genetics. Columbia University Press, New York. 512 pp
Pannacciulli, F. G., D. D. Bishop & S. J. Hawkins, 1997. Genetic structure of populations of two species of Chthamalus (Crustacea: Cirripedia) in the north-east Atlantic and Mediterranean. Mar. Biol. 128: 73–82.
Posada, D. & K. A. Crandall, 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818.
Quesada, H., C. Zapata & G. Alvarez, 1995. A multilocus allozyme discontinuity in the mussel Mytilus galloprovincialis: the interaction of ecological and life-history factors. Mar. Ecol. Prog. Ser. 116: 99–115.
Ramos, A. A., 1988. Ascidias litorales del Mediterráneo Ibérico: faunística, ecología y biogeografía. Ph.D. Thesis. University of Alicante. 405 pp.
Rozas, J. & R. Rozas, 1999. DnaSP version 3.0: an integrated program for molecular population genetics and molecular evolution analyses. Bioinformatics 15: 174–175.
Saavedra, C., C. Zapata, A. Guerra & G. Alvarez G. 1993. Allozyme variation in European populations of the Ostrea edulis. Mar. Biol. 115: 85–95.
Schneider, S., D. Roessli & L. Excoffier. 2000. Arlequin: a software for population genetics data analysis. Ver 2000. Genetics and Biometry Lab., Dept. of Anthropology, University of Geneva. 111 pp.
Svane, I. & C. M. Young. 1989. The ecology and behaviour of ascidian larvae. Oceanogr. mar. biol. Ann. Rev. 27: 45–90.
Swofford, D. L., 1998. PAUP*: Phylogenetic Analysis using Parsimony and other Methods. Sinauer Associates, Sunderland.
Tarjuelo, I., 2001. Reproductive strategies in colonial ascidians: relationships with other life-history traits and genetic structure. Ph.D. Thesis. University of Barcelona. 159 pp.
Tarjuelo, I., D. Posada, K. A. Crandall, M. Pascual & X. Turon, 2001. Cryptic species of Clavelina (Ascidiacea) in two different habitats: harbours and rocky littoral zones in the northwestern Mediterranean. Mar. Biol. 139: 455–462.
Turon, X., 1987. Estudio de las ascidias de las costas de Cataluña e Islas Baleares. Ph.D. Thesis. University of Barcelona. 353 pp.
Vazquez, E., 1993. Estudio faunístico, autoecológico y biogeográfico de los tunicados bentónicos de la ria de ferrol (Galicia). Ph.D. Thesis. University of Santiago de Compostela. 443 pp.
Wirtz, P. & H. R. Martins, 1993. Notes on some rare and little known marine invertebrates from the Azores, with a discussion of the zoogeography of the region. Arquipélago 11A: 55–63.
Wirtz, P., 1998. Twelve invertebrate and eight fish species new to the marine fauna of Madeira, and a discussion of the zoogeography of the area. Helg. Meeres. 52: 197–207.
Wright, S., 1951. The genetical structure of populations. Ann. Eugenics 15: 323–352.
Zibrowius, H., 1991. Ongoing modification of the Mediterranean marine fauna and flora by the establishment of exotic species. Mésogée 51: 83–107.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Turon, X., Tarjuelo, I., Duran, S. et al. Characterising invasion processes with genetic data: an Atlantic clade of Clavelina lepadiformis (Ascidiacea) introduced into Mediterranean harbours. Hydrobiologia 503, 29–35 (2003). https://doi.org/10.1023/B:HYDR.0000008481.10705.c2
Issue Date:
DOI: https://doi.org/10.1023/B:HYDR.0000008481.10705.c2