Abstract
Melatonin is considered as the time-keeping hormone acting on important physiological functions of teleosts. While the influence of melatonin on reproduction and development is well described, its potential role on immune functions has little been considered. In order to better define an immune modulation by the melatonin hormone, we hypothesized that natural variations of photoperiod and subsequent changes in melatonin release profile may act on immune status of pikeperch. Therefore, we investigated during 70 days the effects of two photoperiod regimes simulating the fall and spring in western Europe, on pikeperch physiological and immune responses. Samples were collected at 04:00 and 15:00 at days 1, 37 and 70. Growth, plasma melatonin levels, innate immune markers and expression of immune-relevant genes in head kidney tissue were assessed. While growth and stress level were not affected by the seasonal simulated photoperiods, nocturnal levels of plasma melatonin were photoperiod-dependent. Innate immune markers, including lysozyme, complement, peroxidase and phagocytic activities, were stimulated by the fall-simulated photoperiod and a significant correlation was made with plasma melatonin. In addition to bring the first evidence of changes in fish immunocompetence related to photoperiod, our results provide an additional indication supporting the immunomodulatory action of melatonin in teleosts.
Similar content being viewed by others
Introduction
As photoperiod transducer, the melatonin hormone is mainly produced and secreted by the pineal gland during the night1,2,3,4,5. Through this activity, the pineal gland converts light information into a melatonin signal and thus relays information such as the time of the day and year for cells6,7. The melatonin hormone is indeed seen as the main actor for anticipating changes in season since its peak of production and release by the pineal organ is directly proportional to the length of the night and thus provides a direct transduction of night length8. In mammals as well as in teleosts, melatonin is described to act on important physiological functions, including development and reproduction3,9,10,11,12,13. In mammals, it is also known to interact with the immune system4,12,14,15,16,17,18. However, such immunomodulatory effects of melatonin have little been investigated in fish. Nevertheless, the available information supports an immune regulation by the melatonin hormone in teleosts3,19,20,21,22. In pike perch, a potential dual action of cortisol and melatonin hormones on immune defenses was described23,24. In addition, these experiments defined a correlation between daily cyclic activities of humoral innate immune markers and the nocturnal peak of plasma melatonin.
The life of the organisms is strongly conditioned by seasons, and this influence is more and more marked further from the equator. Seasonality is known to modulate reproductive activity and to influence food intake, locomotor activity, growth performance and immune responses of teleosts8,25,26. In temperate latitudes, the main factors characterizing the seasonal cycle are photoperiod and temperature. From these two factors, the annual cycle of changing photoperiod is the most precise temporal cue for determining the time of year and it has already been well established to influence growth, feeding, smoltification and reproduction8,25,27,28. Photoperiod manipulation is also used in aquaculture to modulate sexual maturation and growth of various fish species29, including the Eurasian perch (Perca fluviatilis) and the pike perch (Sander lucioperca)30. However, the available information of such influence on fish immune system is very scarce. Considering the potential immunomodulatory action of the melatonin hormone and the annual rhythmicity of melatonin secretion by the pineal gland, it is feasible that changing photoperiod co-ordinates fish immunity through the modulation of melatonin secretion. Since bi-directional communications are described in teleosts between HPI axis and both melatonin axis and immune system, several stress markers were considered in the present experiment. While cortisol is known as a potent immunosuppressive agent in vertebrates31,32, a mutual inhibition was described between HPI and melatonin axes33,34,35. In addition, brain serotonergic and dopaminergic activities, which are both indicators of acute and chronic stress in teleosts, were affected by light color and intensity but no consideration of the photoperiod was made24,35,36,37,38.
Pike perch is the most promising freshwater fish species for the diversification of inland aquaculture industry in Europe. The eyes of this species possess a tapetum lucidum that is a specific tissue of the retina which greatly amplify the eye sensitivity to light24,39. This is in agreement with behavior of pike perch since it is a crepuscular predator actively feeding during dusk and night40,41. Previous experiments have defined a high sensitivity of this species to the light environment. Both light intensity and light spectrum were defined as determining factors affecting its physiology, including endocrine and immune functions23,40,42,43. However, as the third light characteristic, photoperiod and its potential effects on fish immunity have still not been considered.
In order to better define in fish the effects of photoperiodic changes on the immunocompetence and the potential key role of the melatonin hormone in this regulation, this study investigated in pike perch the effects of two photoperiod regimes simulating the fall and the spring in western Europe.
Results
Final body weight (282.8 ± 33.5; 276.5 ± 36.3 g) and specific growth rate (1.04 ± 0.28; 1.12 ± 0.37% d−1 for the fall and spring-simulated photoperiod, respectively) did not show any difference between experimental groups. At D70, while the tested photoperiods did not influence the gonadosomatic index for females (0.30 ± 0.04%), a significant difference for males was detected with increased gonadal development with the fall-simulated photoperiod (FSP) (0.41 ± 0.17%) compared to the spring-simulated photoperiod (SSP) (0.22 ± 0.09%). No correlation between immune markers and sexes or gonadosomatic index was tested significant.
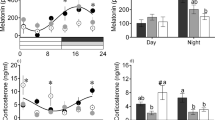
About the tested stress markers, a significant day-night variation was observed for plasma cortisol (p < 0.001), with values reaching 28 ± 4 and 14 ± 2 ng mL−1 during the dark and the light phases respectively (Fig. 1). For plasma glucose (Fig. 1), the highest values were observed for the SSP at D70 (4:00) while the lowest were detected for the FSP at D1 (15:00) (p < 0.05). While no effects were observed concerning the dopaminergic activity (Fig. 2), the serotonergic activity was influenced by the time of the day with increases during the light phase (p < 0.05). The SSP also led to an increase in 5HIAA / 5HT ratio from D1 to D37 (p < 0.05) (Fig. 2).
Day-night (DP: dark phase; LP: light phase) variations of plasma cortisol (left) and glucose (right) in pikeperch juveniles exposed during 70 days to photoperiods simulating the fall or the spring in western Europe. Data are expressed as means ± SEM (n = 12). Capital letters indicate significant differences at p < 0.05. Stars (*) and (***) indicate main effects (day of sampling or time of the day) significant at p < 0.05 and 0.001, respectively.
Day-night (DP: dark phase; LP: light phase) variations of brain serotonergic (left) and dopaminergic (right) activities in pikeperch juveniles exposed during 70 days to photoperiods simulating the fall or the spring in western Europe. Data are expressed as means ± SEM (n = 12). Capital letters indicate significant differences at p < 0.05. Stars (*) indicate main effect (time of the day) significant at p < 0.05.
While constant during the light phase, plasma melatonin values were significantly influenced by the seasonal simulated photoperiods during the night. It showed a progressive increase for the FSP from 89 ± 14 at D1 to 125 ± 18 pg mL−1 at D70 and a decrease for the SSP from 88 ± 17 to 68 ± 14 pg mL1 (Fig. 3). No difference was detected between sexes.
Peroxidase and lysozyme activities in plasma and phagocytic activity in spleen all followed a day-night variation with increased activities at 4:00 whatever the simulated photoperiod (p < 0.001) (Fig. 4a,b,d). The opposite scheme was observed for plasma complement activity (Fig. 4c) with the highest values detected at 15:00 (p < 0.001). In addition, lysozyme, peroxidase and complement activities were significantly influenced by the simulated photoperiod with increases observed from D1 to D70 for the FSP. Furthermore, the lysozyme activity significantly decreased from D1 (15:00) to D70 (15:00) for the SSP (p < 0.05). An increase in phagocytic activity was also observed at D37 (p < 0.05) for both photoperiods (Fig. 4d). Significant positive correlations between night variations of plasma melatonin and immune markers were tested significant, including for lysozyme (correlation: 0.41; p < 0.001), peroxidase (0.28; p < 0.01) and complement (0.38; p < 0.01).
Day-night (DP: dark phase; LP: light phase) variations of (a) lysozyme, (b) peroxidase and (c) hemolytic complement activities in plasma and (d) phagocytic activity in spleen of pikeperch juveniles exposed during 70 days to photoperiods simulating the fall or the spring in western Europe. Data are expressed as means ± SEM (n = 15). Capital letters indicate significant differences at p < 0.05. Stars (***) indicate main effect (time of the day) significant at p < 0.001.
Considering expression of immune-related genes, no effects were detected on il-1 and hepc (Fig. 5a,b). However, c3 gene expression increased at D70 for the FSP (P < 0.001) (Fig. 5c). In addition, an increase in tnf-α gene expression was detected at D70 whatever the experimental group while a decrease in lys gene expression was observed at D70 compared to D37 (Fig. 5d,e).
Day-night (DP: dark phase; LP: light phase) variations of (a) il-1, (b) hepc, (c) c3, (d) tnf-α and (e) lys gene expression in head kidney of pikeperch juveniles exposed during 70 days to photoperiods simulating the fall or the spring in western Europe. Data are expressed as means ± SEM (n = 12). Capital letters indicate significant differences at p < 0.05. Stars (*), (**) and (***) indicate main effects (day of sampling or time of the day) significant at p < 0.05, 0.01 and 0.001, respectively.
Discussion
Seasonality, that is mainly characterized by variations of both temperature and day length, is described to affect immune responses of vertebrates, including fish, reptiles, birds and mammals25,26,44,45,46. Thus seasonal variations of several innate immune markers, including complement, peroxidase and lysozyme activities, have been observed in sea bass (Dicentrarchus labrax), dab (Limanda limanda), halibut (Hippoglossus hippoglossus) and Asian catfish (Clarias batrachus)47,48,49,50. Temperature was described several times to be the main seasonal factor operating on fish immune system since lower temperatures lead to a shutdown or slowing of immune mechanisms leading to an increase in fish susceptibility to disease50,51,52. However, the study of Valero et al.50 has reported that seasonal variations of some immune markers are not related with the temperature and that they could be explained by daylight changes, supporting that both temperature and photoperiod variations act significantly on fish immune system throughout the year. Nevertheless, to our knowledge, no studies conducted specifically on photoperiod in a seasonal context has been published until now. Several studies have focused on photoperiod manipulation (extreme light regimes including constant light or darkness). This practice, that has become common in the aquaculture industry to obtain out-of-season reproductions25, affects the levels of stress hormones in rainbow trout (Oncorhynchus mykiss), which consequently alter immune functions29. It was also shown to affect larval development and survival of European sea bass and Senegalese sole53,54.
In the present experiment, the season-simulated photoperiod regimes did not influence growth parameters. Since seasonality was shown to highly influence growth of various fish species25,49, our results suggest that such effect is mainly driven by temperature and/or food availability. In addition, values of stress markers suggest that natural gradual changes in L:D are not stressful for pike perch and that it should still be considered in management strategies of fish culture. Since tryptophan is a precursor of both melatonin and serotonin hormones35, the daily rhythm in 5HIAA/5-HT ratio may be the result of a decrease in tryptophan availability during the dark phase of the photoperiod due to melatonin synthesis.
An early gonadogenesis was observed at day 70 for both sexes, with the highest development for males maintained under the FSP. The decrease in day length is involved in the initiation of gametogenesis in many fish species including the walleye (Sander vitreus)55 and the Eurasian perch56. Our results are also consistent with results from Ben Ammar et al.57 showing that the initiation of gonadogenesis seems to be mainly driven by photoperiodic changes. However, both temperature and photoperiod variations must be considered in the control of pikeperch reproductive cycle57,58.
A potential effect of such gonadal development on the immune system cannot be discarded since interactions between neuroendocrine and immune systems are widely accepted59,60. Moreover, season-dependent changes in fish immune system were shown to be correlated with changes in the levels of circulating sex hormones61. For example, phagocytosis and lysozyme activities, respiratory burst activity in blood leukocytes as well as ROS and NOS production are all influenced by estrogens in various fish species. However, in the present experiment, the correlation study does not highlight any relation between immune markers and gonadal development, supporting that immune status of young pike perch may be only slightly influenced by the initiation of gonadogenesis.
We showed for the first time the variations of immune markers according to the season-simulated photoperiod. Lysozyme, complement and peroxidase activities, as well as c3 gene expression in head kidney, all increased following the decrease in day length during 70 days. In contrast, only lysozyme showed a decrease under the spring-simulated photoperiod. In addition, the correlation study support that variations of innate immune markers are related to the nocturnal variations in plasma melatonin. The progressive exposure to shorter photoperiods leads to an increase in melatonin production and release by the pineal gland since its activity is directly proportional to the length of the night8. Few evidences have suggested that the melatonin hormone may act as an important immune regulator in teleosts3,20. For instance, several immune markers including peroxidase, phagocytic ability and cytotoxic activity of head kidney leucocytes as well as the expression of immune genes in head kidney (il-1β, major histocompatibility complex, and interferon-regulatory factor-1) are increased in gilthead seabream (Sparus aurata) following intraperitoneal injection of melatonin20. And, in zebrafish, endogenous melatonin enhances neutrophil migration following increase in cytokine expressions such as Il-8 and Il-1β22.
Our results defined also day-night variations of lysozyme, peroxidase, complement and phagocytic activities. Such cyclic activity has already been described in several fish species including pike perch, Nile tilapia (Oreochromis niloticus), gilthead seabream and sea bass19,23,24,62. The day-night variations of immune variables, as observed in the present experiment, are comparable to the variations of both plasma melatonin and cortisol23,24. Considering that cortisol is a potent immunosuppressive agent with complex actions on immune cells and tissues31,32 and that an anti-stress role for melatonin has been defined33,35, the present results may support that melatonin acts on immune activities through the modulation of cortisol release in plasma3,63. However, cortisol peak was described to occur at different times of the day according to the experiment and the fish species, supposing that it is, at least partially, light- and melatonin-independent. Innate immune markers are also differently stimulated according to the time of the day. Even if the underlying mechanisms are still not well understood and that differences of cyclic pattern are observed among fish species19,23,24, there are always one or several innate immune activities enhanced whatever the time of the day, ensuring a constant immune protection against pathogens.
All in all, the daily rhythms of innate immune markers and the up- and down-regulations according to the photoperiod support a significant effect of the melatonin hormone on the innate immune system of fish. However, the model of action still needs investigations. Melatonin could act on immune cells and tissues indirectly through the regulation of several hormones (glucocorticoids, growth hormone, prolactin…) or through specific melatonin receptors3. In several teleost species, three high-affinity receptor subtypes were identified, including MT1, MT2 and Mel1c64. While several studies focused on melatonin receptor distribution65,66,67,68, few has examined their presence in immune cells or tissues. They are found in the kidney and the spleen of several fish species66,68,69 but no study has investigated the exact location of these receptors in the immune tissues neither their functional significance.
In addition, the present study did not examine the potential role of an internal clock machinery in immune modulation according to seasonal simulated photoperiods. Organisms, including fish, show circadian rhythms (repeating roughly every 24 h) of activity, food intake, body change color, oxygen consumption and some physiological parameters3,70. These rhythms are under the control of different environmental cues, with the light being the strongest of these synchronizers. Other environmental parameters changing daily or annually, including temperature and food availability, are also critical70. The main characteristic of these circadian rhythms is the persistence of their oscillations for a certain period of time, even in the absence of these environmental cues, by being driven by a circadian clock3. In zebrafish, a complex network of coexisting central and peripheral clocks was described and peripheral tissue pacemakers, that have been identified in several extra-retinal/extra-pineal tissues, were shown to be directly responsive to light71. However, while several studies described daily variations of immune activities19,23, none has investigated in fish, and to our knowledge, circadian activity of the immune system or peripheral clock in immune tissues. The potential presence of such pacemaker in immune tissue should be investigated in order to better describe the relationship between the photoperiod, the clock machinery and the immune system.
In conclusion, this study showed for the first time in a teleost fish an innate immune modulation according to the seasonal and daily variations of photoperiod. As the time-keeping hormone, melatonin is seen as one of the main mediator acting on fish immune system. Such regulation may involve both direct and indirect action of melatonin on immune targets. Better consideration of the light environment is suggested to improve immunocompetence of cultured fish species and to limit disease outbreaks.
Material and Methods
Animals and rearing conditions
The experiment was carried out at the Aquaculture Experimental Platform (AEP, registration number for animal experimentation C54-547-18) belonging to the URAFPA lab and located at the Faculty of Sciences of the University of Lorraine (France). All experimental manipulations were carried out in agreement with the European and French national legislations on animal welfare after evaluation and approval of the experimental project (protocol number: APAFIS10285-201706201445413) by the local ethic committee in France (Name: CELMEA; French code: 066). A stock of 1,500 mixed-sex pike perch juveniles was provided by Asialor farm (Dieuze, France) and transferred to the facilities. Animals were randomly distributed into 12 indoor 2000-L tanks. Each of these 12 experimental units was operating independently in a recirculating system (RAS). Fish were acclimated for 29 days in constant conditions (temperature: 21 °C; light intensity: 15 lx; photoperiod: LD 12:12) and fed once daily at 2% biomass. They reached 149 ± 21 g at the first day of the experiment. In order to simulate the fall and the spring light conditions in Western Europe, gradual changes in LD from 12(8:00–20:00):12 to 8(10:21–18:16):16 or 12(8:00–20:00):12 to 16(7:25–23:30):8, respectively, were set up for 70 days according to natural photoperiods in Paris, France. Additionally, a dusk and a dawn of 30 min were programmed. Every other rearing condition was maintained constant during all the 70-day experiment. In order to limit high size heterogeneity due to a high social dominance observed in pike perch, fish were fed once a day during the light phase, 2.5 h after sunrise.
Sampling procedures
Samplings at days 1, 37 and 70 occurred during scotophase at 04:00 h and photophase at 15:00 h. To avoid stress artefacts of nocturnal fishing on diurnal samplings, the number of tanks was doubled. Each of the 4 treatment groups, considering the two simulated photoperiods and the two sampling times, had thus 3 replicates. Five fish were removed randomly from each tank and anesthetized with MS-222 (150 mg L−1). Blood was quickly collected by caudal vein puncture with heparinized syringes within 4 min and centrifuged at 3,000 g during 10 min at 4 °C. Fish were then euthanized before collecting the spleen, the whole brain and the anterior kidney. Plasma, brain and anterior kidney were directly frozen in liquid nitrogen and stored at −80 °C until assayed. Spleen Spleen was stored on ice in L-15 media.
Final individual weight and specific growth rate were determined on day 70 for each experimental condition. Specific growth rate was estimated according to the formula: ((Ln (final individual weight) – Ln (initial individual weight)) *100/duration of the experiment). Mortality was recorded along the whole experiment.
Since a slight gonadal development was observed for both males and females at D70, the gonadosomatic index (%) was estimated according to the formula: (gonad weight * 100/body weight).
Stress indicators
Plasma cortisol and glucose
Cortisol was assayed in triplicate using a cortisol ELISA kit (DRG, EIA-1887), following the manufacturer’s instructions (BioSource, Belgium). The intra-assay coefficient of variation was 3.6%, the assay dynamic range was between 0–800 ng mL−1 and the analytical sensitivity was 2.5 ng mL−1 23. Plasma glucose, also assayed in triplicate, was determined calorimetrically based on a glucose oxidase/peroxidase method described by Trinder23,72.
Brain neurotransmitters
High Performance Liquid Chromatography (HPLC) was performed according to the methods of Lepage et al.73, with some modifications24, to assess in whole brain the serotonergic and dopaminergic activities expressed as hydroxyl-indol-acetic acid (5-HIAA)/serotonin (5-HT) and 3,4-dihydroxyphenylacetic acid (DOPAC)/dopamine (DA) ratios, respectively.
Melatonin content in plasma
As described in Baekelandt et al.24, plasma melatonin was assayed in triplicate using a Melatonin ELISA kit (E-EL-M0788, Elabscience Biotechnology Co., USA), following the manufacturer’s instructions. Recovery rate was estimated around 90 to 95% for melatonin values ranging from 5 to 100 pg mL−1. Intra and inter-assays of coefficients were 5.8 and 7.4%, respectively (n = 4). Nocturnal plasma samples were diluted to get values between 5 and 100 pg mL−1.
Humoral immune variables
The total peroxidase activity in plasma was assessed following the method described in Quade and Roth74. One unit of peroxidase activity corresponds to an absorbance change of 1 OD.
The alternative complement pathway (ACH50), as described in Baekelandt et al.23, was assayed by measuring the haemolytic activity in plasma samples using rabbit erythrocytes as targets75. Briefly, 10 µL of rabbit red blood cells suspension suspended at 3% in veronal buffer (Biomerieux, Marcy-l’Etoile, France) were mixed with serial dilutions of plasma (from 40 to 800 times). Plates were then read at 615 nm after incubation at 28 °C for 120 min. The spontaneous hemolysis was obtained by adding veronal buffer to 10 µL of rabbit erythrocytes and total lysis was obtained by mixing 10 µL of rabbit erythrocytes to distilled water (total volume = 70 µL). ACH50 corresponds to the lysis of 50% of the rabbit erythrocytes.
Lysozyme activity was evaluated in plasma samples by the turbimetric method76,77. Lysozyme activity (units) is defined as the amount of enzyme decreasing the turbidity of 0.001 OD per min.
Phagocytic activity
Spleen tissues were washed once with L-15 medium. They were then gently mashed with 9 mL of L15 medium supplemented with bovine serum albumin (10%, Sigma-Aldrich) and Penicillin– streptomycin (P/S) (1%, Sigma-Aldrich) through a 100 µm nylon mesh grid. Cell suspensions were kept at 4 °C for 16 h and then centrifuged and washed twice with L15 medium. Cells were suspended in 1 mL of L-15 medium containing 1% of P/S.
Cell mortality was assessed by flow cytometry with a FACSVerse (BD Biosciences, USA) using propidium iodide (PI) probe (1 μg mL−1). Samples were considered for analysis when survival exceeded 90%. For the phagocytosis assay, 1 × 106 cells were incubated with yellow-green fluorescent latex beads (Fluoresbrite®, Polyscience; 2 µm diameter) for 18 h at 21 °C with a 1/100 ratio cell-beads ratio. Non-ingested beads were eliminated following a centrifugation step (400 × g, 10 min, 4 °C). Cells were fixed with a 0.5% formaldehyde and 0.2% sodium azide PBS fixating solution. The phagocytic activity (percentage of cells that have ingested three or more beads) was measured through cytometric analysis78.
Gene expression analysis
Total RNA isolation from anterior kidney tissue, which is important in hematopoiesis and immunity in fish, was performed using Extract-all® Reagent (Eurobio) following manufacturer’s instructions and description in Baekelandt et al.24. Each RNA sample was subjected to DNase treatment (DNase Ambion; Life Technologies) and reverse-transcription (RevertAid™ H Minus First Strand cDNA Synthesis Kit; Thermo Scientific) following the manufacturer’s instructions. The relative expression of several immune-related genes was investigated by RT-qPCR, including genes involved in bactericidal defense, namely C-type lysozyme (lys), hepcidin c (hepc), and complement C3 (c3), and in pro-inflammatory action, namely interleukin-1 (il-1) and tumor necrosis factor alpha (tnf-α). In addition, expression of reference genes β-actin and elongation factor alpha (ef1-α), whose expressions were tested stable in experimental conditions, were assessed. Efficiencies of primers (Table 1)24 were validated when ranged between 90 and 105%. The relative mRNA levels of c3, lys, il-1, hepc, and tnf-α in each sample were normalized with the geometric mean of ef1-α and β-actin calculated by the relative standard curve method79.
Statistical analysis
Data are expressed as the mean ± standard error (SEM). Kolmogorov and Smirnov’s test was used to assess the normality of data sets (p < 0.05) and Bartlett’s test was conducted to evaluate variance homogeneity (p < 0.05). Logarithmic transformations were made to achieve normality and homoscedasticity when necessary. Results were analyzed with a three-way ANOVA (p < 0.05) taking the photoperiod regime (fall and spring), the day of sampling (D1, D37 and D70) and the time of the day (4:00 and 15:00) as factors. Statistics were performed using the fish as the experimental unit with the exception of growth parameters (final individual weight and specific growth rate). Tank effect was previously tested not significant. When interactions were tested significant, values were compared according to Tukey’s HSD post-hoc test (p < 0.05). In addition, correlations between immune markers and gonadosomatic index as well as between immune activities and night variations of plasma melatonin were tested for significance. The results were analyzed with JMP 12.1 software (SAS Institute Inc., North Carolina, USA) and graphs were performed with GraphPad Prism V5.04 (California, USA).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Migaud, H. & Al, E. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm. J. Pineal Res. 41, 42–52 (2006).
Migaud, H., Davie, A., Martinez Chavez, C. C. & Al-Khamees, S. Evidence for differential photic regulation of pineal melatonin synthesis in teleosts. J. Pineal Res. 43(4), 327–335 (2007).
Falcón, J., Migaud, H., Muñoz-Cueto, J. A. & Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 165(3), 469–482 (2010).
Pierozan, P., Andersson, M. & Karlsson, I. O. The environmental neurotoxin β-N‐methylamino‐L‐alanine inhibits melatonin synthesis in primary pinealocytes and a rat model. J. Pineal Res. 65(1), e12488 (2018).
Xia, Y. et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 66(2), e12547 (2018).
Kulczykowska, E. & Sánchez-Vázquez, F. J. Neurohormonal regulation of feed intake and response to nutrients in fish: Aspects of feeding rhythm and stress. Aquac. Res. 41(5), 654–667 (2010).
Brüning, A., Hölker, F., Franke, S., Preuer, T. & Kloas, W. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Sci. Total Environ. 511, 516–522 (2015).
Bromage, N., Porter, M. & Randall, C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture. 197, 63–98 (2001).
Downing, G. & Litvak, M. K. Effects of light intensity, spectral composition and photoperiod on development and hatching of haddock (Melanogrammus aeglefinus) embryos. Aquaculture. 213, 265–278 (2002).
Vera, L. M. et al. Seasonal and daily plasma melatonin rhythms and reproduction in Senegalese sole kept under natural photoperiod and natural or controlled water temperature. J. Pineal Res. 43, 50–55 (2007).
Mehner, T. Diel vertical migration of freshwater fishes - proximate triggers, ultimate causes and research perspectives. Freshwater Biol. 57(7), 1342–1359 (2012).
Carrillo-Vico, A., Guerrero, J. M., Lardone, P. J. & Reiter, R. J. A review of the multiple actions of melatonin on the immune system. Endocrine. 27(2), 189–200 (2005).
Dumbell, R., Matveeva, O. & Oster, H. Circadian clocks, stress, and immunity. Front Endocrinol. 7, 1–8 (2016).
Calvo, J. R., Mohammed, R. E. I., Pozo, D. & Guerrero, J. M. Immunomodulatory role of melatonin: specific binding sites in human and rodent lymphoid cells. J. Pineal Res. 18, 119–126 (1995).
Pozo, D., Garcia-Maurino, S., Guerrero, J. M. & Calvo, J. R. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J. Pineal Res. 37, 48–54 (2004).
Peña, C., Rincon, J., Pedreanez, A., Viera, N. & Mosquera, J. Chemotactic effect of melatonin on leukocytes. J. Pineal Res. 43, 263–269 (2007).
Pontes, G. N., Cardoso, E. C., Carneiro-Sampaio, M. M. & Markus, R. P. Pineal melatonin and the innate immune response: the TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res. 43, 365–371 (2007).
Hardeland, R. Melatonin and inflammation - Story of a double-edged blade. J. Pineal Res. 65(4), e12525 (2018).
Esteban, M. A., Cuesta, A., Rodriguez, A. & Meseguer, J. Effect of photoperiod on the fish innate immune system: a link between fish pineal gland and the immune system. J. Pineal Res. 41(3), 261–266 (2006).
Cuesta, A., Cerezuela, R., Esteban, M. A. & Meseguer, J. In vivo actions of melatonin on the innate immune parameters in the teleost fish gilthead seabream. J. Pineal Res. 45(1), 70–78 (2008).
Esteban, M. A., Cuesta, A., Chaves-Pozo, E. & Meseguer, J. Influence of melatonin on the immune system of fish: a review. Int. J. Mol. Sci. 14, 7979–7999 (2013).
Ren, D. I., Ji, C., Wang, X. B., Wang, H. & Hu, B. Endogenous melatonin promotes rhythmic recruitment of neutrophils toward an injury in zebrafish. Sci. Rep. 7, 4696 (2017).
Baekelandt, S., Mandiki, S., Schmitz, M. & Kestemont, P. Influence of the light spectrum on the daily rhythms of stress and humoral innate immune markers in pikeperch Sander lucioperca. Aquaculture. 499, 358–363 (2019).
Baekelandt, S., Mandiki, S. N. M. & Kestemont, P. Are cortisol and melatonin involved in the immune modulation by the light environment in pikeperch Sander lucioperca? J. Pineal Res. 67, e12573 (2019b).
Bowden, T. J., Thompson, K. D., Morgan, A. L., Gratacap, R. M. L. & Nikoskelainen, S. Seasonal variation and the immune response: A fish perspective. Fish Shellfish Immunol. 22(6), 695–706 (2007).
Morgan, A. L., Thompson, K. D., Auchinachie, N. A. & Migaud, H. The effect of seasonality on normal haematological and innate immune parameters of rainbow trout Oncorhynchus mykiss L. Fish Shellfish Immunol. 25, 791–799 (2008).
Nelson, R. J. & Demas, G. E. Role of melatonin in mediating seasonal energetic and immunologic adaptations. Brain Res. Bull. 44, 423e30 (1997).
Nelson, R.J., Demas, G.E., Klein, S.L. & Kriegsfeld, L.J. Seasonal patterns of stress, immune function, and disease. Cambridge University Press, New-York (2002).
Leonardi, M. O. & Klempau, A. E. Artificial photoperiod influence on the immune system of juvenile rainbow trout (Oncorhynchus mykiss) in the Southern Hemisphere. Aquaculture. 221, 581–591 (2003).
Fontaine, P., Wang, N. & Hermelink, B. Broodstock management and control of the reproductive cycle. In: Kestemont, P., Dabrowski, K. & Summerfelt, R. C. (Eds.), Biology and culture of Percid fishes. Springer Science+Business Media Dordrecht, pp. 61–100 (2015).
Laiz-Carrión, R., Martín Del Río, P., Miguez, J. M., Mancera, J. M. & Soengas, J. L. Influence of cortisol on osmoregulation and energy metabolism in Gilthead Seabream. Sparus aurata. J. Exp. Zool. 298A, 105–118 (2003).
Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 35, 1366–1375 (2011).
López-Patiño, M. A. et al. Melatonin partially minimizes the adverse stress effects in Senegalese sole (Solea senegalensis). Aquaculture. 388-391(1), 165–172 (2013).
López-Patiño, M. A., Gesto, M., Conde-Sieira, M., Soengas, J. L. & Miguez, J. M. Stress inhibition of melatonin synthesis in the pineal organ of rainbow trout (Oncorhynchus mykiss) is mediated by cortisol. J. Exp. Biol. 217(8), 1407–1416 (2014).
Conde-Sieira, M. et al. Oral administration of melatonin counteracts several of the effects of chronic stress in rainbow trout. Domest. Anim. Endocrinol. 46, 26–36 (2014).
Karakatsouli, N. et al. Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquac. Eng. 36(3), 302–309 (2007).
Gesto, M., López-Patiño, M. A., Hernández, J., Soengas, J. L. & Míguez, J. M. The response of brain serotonergic and dopaminergic systems to an acute stressor in rainbow trout: a time course study. J. Exp. Biol. 216, 4435–4442 (2013).
Gesto, M. et al. A simple melatonin treatment protocol attenuates the response to acute stress in the sole Solea senegalensis. Aquaculture. 452, 272–282 (2016).
Feiner, Z.S. & Höök, T.O. Environmental biology of Percid fishes. In: Kestemont, P., Dabrowski, K. & Summerfelt, R. C. (Eds.), Biology and culture of Percid fishes. Springer Science+Business Media Dordrecht, pp. 61–100 (2015).
Luchiari, A. C., Freire, F., Koskela, J. & Pirhonen, J. Light intensity preference of juvenile pikeperch Sander lucioperca (L.). Aquac. Res. 37, 1572–1577 (2006).
Dalsgaard, J. et al. Farming different species in RAS in Nordic countries: current status and future perspectives. Aquac. Eng. 53, 2–13 (2013).
Luchiari, A. C., Freire, F., Pirhonen, J. & Koskela, J. Longer wavelengths of light improve the growth, intake and feed efficiency of individually reared juvenile pikeperch Sander lucioperca (L.). Aquac Res. 40, 880–886 (2009).
Baekelandt, S. et al. Multifactorial analyses revealed optimal aquaculture modalities improving husbandry fitness without clear effect on stress and immune status of pikeperch Sander lucioperca. Gen. Comp Endocrinol. 258, 194–204 (2018).
Zapata, A. G., Varas, A. & Torroba, M. Seasonal variations in the immune system of lower vertebrates. Immunol. Today. 13, 142–147 (1992).
Martin, L. B., Weil, Z. M. & Nelson, R. J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363(1490), 321–339 (2008).
Zimmerman, L. M., Vogel, L. A. & Bowden, R. M. Understanding the vertebrate immune system: insights from the reptilian perspective. J. Exp. Biol. 213, 661–671 (2010).
Bowden, T. J., Butler, R. & Bricknell, I. R. Seasonal variation of serum lysozyme levels in Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 17(2), 129–135 (2004).
Kumari, J. et al. Seasonal variation in the innate immune parameters of the Asian catfish Clarias batrachus. Aquaculture 252, 121127 (2006).
Pascoli, F. et al. Seasonal effects on hematological and innate immune parameters in sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 31, 1081–1087 (2011).
Valero, Y., Garc, A., Cuesta, A. & Chaves-pozo, E. Seasonal variations of the humoral immune parameters of European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 39, 185–187 (2014).
Le Morvan, C., Troutaud, D. & Deschaux, P. Differential effects of temperature on specific and nonspecific immune defenses in fish. J. Exp. Biol. 201, 165–168 (1998).
Abram, Q. H., Dixon, B. & Katzenback, B. A. Impacts of Low Temperature on the Teleost Immune System. Biology (Basel.). 6(4), 39 (2017).
Villamizar, N., García-Alcazar, A. & Sánchez-Vázquez, F. J. Effect of light spectrum and photoperiod on the growth, development and survival of European sea bass (Dicentrarchus labrax) larvae. Aquaculture. 292, 80–86 (2009).
Blanco-vives, B. et al. Effect of daily thermo- and photo-cycles of different light spectrum on the development of Senegal sole (Solea senegalensis) larvae. Aquaculture. 306(1–4), 137–145 (2010).
Malison, J. A., Procarione, L. S., Kayes, T. B., Hansen, J. F. & Held, J. A. Induction of out-of-season spawning in walleye (Stizostedion vitreum). Aquaculture. 163, 151–161 (1998).
Abdulfatah, A., Fontaine, P., Kestemont, P., Gardeur, J.-N. & Marie, M. Effects of photothermal kinetics and amplitude of photoperiod decrease on the induction of the reproduction cycle in female Eurasian perch Perca fluviatilis. Aquaculture. 322–323, 169–176 (2011).
Ben Ammar, I. et al. Continuous lighting inhibits the onset of reproductive cycle in pikeperch males and females. Fish Physiol. Biochem. 41(2), 345–356 (2015).
Hermelink, B., Wuertz, S., Rennert, B., Kloas, W. & Schulz, C. Temperature control of pikeperch (Sander lucioperca) maturation in recirculating aquaculture systems – induction of puberty and course of gametogenesis. Aquaculture. 400-401, 36–45 (2013).
Engelsma, M. Y. et al. Neuroendocrine-immune interactions in fish: a role for interleukin-1. Vet. Immunol. Immunopathol. 87(3-4), 467–479 (2002).
Nardocci, G. et al. Neuroendocrine mechanisms for immune system regulation during stress in fish. Aquaculture. 40(2), 531–538 (2014).
Szwejser, E., Kemenade, B. M. L. V., Maciuszek, M. & Chadzinska, M. Hormones and Behavior Estrogen-dependent seasonal adaptations in the immune response of fish. Horm. Behav. 88, 15–24 (2017).
Lazado, C. C., Skov, P. V. & Pedersen, P. B. Innate immune defenses exhibit circadian rhythmicity and differential temporal sensitivity to a bacterial endotoxin in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 55, 613–622 (2016).
Kulczykowska, E., Kalamarz-Kubiah, H., Gozdowska, M. & Sokolowska, E. Cortisol and melatonin in the cutaneous stress response system of fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 218, 1–7 (2018).
Falcón, M. A., Besseau, L., Sauzet, S. & Bœuf, G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol. Metabol. 18, 81–88 (2007).
Bayarri, M. J. et al. Binding characteristics and daily rhythms of melatonin receptors are distinct in the retina and the brain areas of the European sea bass retina (Dicentrarchus labrax). Brain Res. 1029, 241–250 (2004).
Park, Y. J. et al. Melatonin receptor of a reef fish with lunar-related rhythmicity: cloning and daily variations. J. Pineal Res. 41, 166–174 (2006).
Sauzet, S. et al. Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol. 157, 186–195 (2008).
Confente, F., Rendón, M. C., Besseau, L., Falcón, J. & Muñoz-Cueto, J. A. Melatonin receptors in a pleuronectiform species, Solea senegalensis: Cloning, tissue expression, day-night and seasonal variations. Gen. Comp. Endocrinol. 167(2), 202–214 (2010).
Kulczykowska, E., Kalamarz, H., Warne, J. M. & Balment, R. J. Day-night specific binding of 2[125I]iodomelatonin and melatonin content in gill, small intestine and kidney of three fish species. J. Comp. Physiol. B. 176, 277–285 (2006).
Zhdanova, I.V., Reebs, S.G. Circadian rhythms in fish. In: Sloman, K. A., Wilson, R. W. & Balshine, S. eds. Behaviour and physiology of fish. Academic Press, Vol. 24: 197–238, 2006.
Prokkola, J. M. & Nikinmaa, M. Circadian rhythms and environmental disturbances – underexplored interactions. J. Exp. Biol. 221, jeb179267 (2018).
Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6, 24–27 (1969).
Lepage, O., Overli, O., Petersson, E., Järvi, T. & Winberg, S. Differential Stress Coping in Wild and Domesticated Sea Trout. Brain. Behav. Evol. 56, 259–268 (2000).
Quade, M. J. & Roth, J. A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 58, 239–248 (1997).
Sunyer, J. O. & Tort, L. Natural haemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet. Immunol. Immunopathol. 45(3–4), 333–345 (1995).
Siwicki, A. & Studnicka, M. The phagocytic ability of neutrophils and serum lysozyme activity in experimentally infected carp, Cyprinus carpio L. J. Fish Biol. 31, 57–60 (1987).
Douxfils, J. et al. Physiological and proteomic responses to single and repeated hypoxia in juvenile Eurasian perch under domestication - Clues to physiological acclimation and humoral immune modulations. Fish Shellfish Immunol. 33, 1112–1122 (2012).
Samaï, H. C. et al. Procedures for leukocytes isolation from lymphoid tissues and consequences on immune endpoints used to evaluate fish immune status: A case study on roach (Rutilus rutilus). Fish Shellfish Immunol. 74, 190–204 (2018).
Larionov, A., Krause, A. & Miller, W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 6, 62 (2005).
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the grant agreement No. 652831 (AQUAEXCEL2020). This output reflects only the author’s view and the European Union cannot be held responsible for any use that may be made of the information contained therein. This work was also supported by the FRIA (Fonds de la Recherche dans l’Industrie et l’Agriculture, Wallonia-Brussels Federation), providing a grant to S.B.
Author information
Authors and Affiliations
Contributions
S.B. performed the experiment and several analyses (HPLC, ELISA kits, innate immune activities, gene expression) and wrote the main manuscript text. Y.L. was in charge of fish care. V.C. and E.F. performed phagocytic activity. S.M., S.N.M.M. and P.K. were involved in the design of the study and in the revision of the manuscript. All co-authors assisted on the samplings.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baekelandt, S., Milla, S., Cornet, V. et al. Seasonal simulated photoperiods influence melatonin release and immune markers of pike perch Sander lucioperca. Sci Rep 10, 2650 (2020). https://doi.org/10.1038/s41598-020-59568-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59568-1
This article is cited by
-
Pikeperch (Sander lucioperca) adaptive or maladaptive reactions to environmental stressors؟ a review
Aquaculture International (2024)
-
Insights into early ontogenesis: characterization of stress and development key genes of pikeperch (Sander lucioperca) in vivo and in vitro
Fish Physiology and Biochemistry (2021)
-
Genetic Diversity of SARS-CoV2 and Environmental Settings: Possible Association with Neurological Disorders
Molecular Neurobiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.