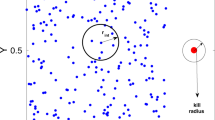
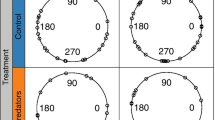
Abstract
We study the cumulative effect of successive predator attacks on the disturbance of a prey aggregation using a modelling approach. Our model intends to represent fish schools attacked by both aerial and underwater predators. This individual-based model uses long-distance attraction and short-distance repulsion between prey, which leads to prey aggregation and swarming in the absence of predators. When intermediate-distance alignment is added to the model, the prey aggregation displays a cohesive displacement, i.e., schooling, instead of swarming. Including predators, i.e. with repulsion behaviour for prey to predators in the model, leads to flash expansion of the prey aggregation after a predator attack. When several predators attack successively, the prey aggregation dynamics is a succession of expanding-grouping-swarming/schooling phases. We quantify this dynamics by recording the changes in the simulated prey aggregation radius over time. This radius is computed as the longest distance of individual prey to the aggregation centroid, and it is assumed to increase along with prey disturbance. The prey aggregation radius generally increases during flash expansion, then decreases during grouping until reaching a constant lowest level during swarming/schooling. This general dynamics is modulated by several parameters: the frequency, direction (vertical vs. horizontal) and target (centroid of the prey aggregation vs. random prey) of predator attacks; the distance at which prey detect predators; the number of prey and predators. Our results suggest that both aerial and underwater predators are more efficient at disturbing fish schools by increasing their attack frequency at such level that the fish cannot return to swarming/schooling. We find that a mix between aerial and underwater predators is more efficient at disturbing a fish school than a single type of attack, suggesting that aerial and underwater foragers may gain mutual benefits in forming foraging groups.







Similar content being viewed by others
References
Au DWK, Pitman RL (1986) Seabird interactions with dolphins and tuna in eastern tropical Pacific. Condor 88(3):304–317. doi:10.2307/1368877
Axelsen BE, Anker-Nilssen T, Fossum P, Kvamme C, Nøttestad L (2001) Pretty patterns but a simple strategy: predator–prey interactions between juvenile herring and Atlantic puffins observed with multibeam sonar. Can J Zool 79(9):1586–1596. doi:10.1139/cjz-79-9-1586
Bainbridge R (1960) Speed and stamina in three fish. J Exp Biol 37(1):129–153
Ballerini M, Calbibbo N, Candeleir R, Cavagna A, Cisbani E, Giardina I, Lecomte V, Orlandi A, Parisi G, Procaccini A, Viale M, Zdravkovic V (2008) Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc Natl Acad Sci U S A 105(4):1232–1237. doi:10.1073/pnas.0711437105
Boesch C (1994) Cooperative hunting in wild chimpanzees. Anim Behav 48(3):653–667. doi:10.1006/anbe.1994.1285
Boyd IL, Arnould JPY, Barton T, Croxall JP (1994) Foraging behavior of Antartic fur seals during periods of contrasting prey abundance. J Anim Ecol 63(3):703–713. doi:10.2307/5235
Camperi M, Cavagna A, Giardina I, Parisi G, Silvestri E (2012) Spatially balanced topological interaction grants optimal cohesion in flocking models. Interface Focus 2(6):715–725. doi:10.1098/rsfs.2012.0026
Camphuysen CJ, Webb A (1999) Multi-species feeding associations in North Sea seabirds: jointly exploiting a patchy environment. Ardea 87(2):177–198
Clua E, Grosvalet F (2001) Mixed-species feeding aggregation of dolphins, large tunas and seabirds in the Azores. Aquat Living Resour 14(1):11–18. doi:10.1016/s0990-7440(00)01097-4
Coetzee JC, Merkle D, Hutchings L, van der Lingen CD, van den Berg M, Durholtz MD (2010) The 2005 KwaZulu-Natal sardine run survey sheds new light on the ecology of small pelagic fish off the east coast of South Africa. Afr J Mar Sci 32(2):337–360. doi:10.2989/1814232x.2010.502639
Coleman RA, Browne M, Theobalds T (2004) Aggregation as a defense: limpet tenacity changes in response to simulated predator attack. Ecology 85(4):1153–1159. doi:10.1890/03-0253
Creel S, Creel NM (1995) Communal hunting and pack size in African wild dogs, Lycaon Pictus. Anim Behav 50:1325–1339. doi:10.1016/0003-3472(95)80048-4
Dejean A, Fénéron R (1999) Predatory behaviour in the ponerine ant, Centromyrmex bequaerti: a case of termitolesty. Behav Process 47(2):125–133. doi:10.1016/s0376-6357(99)00060-1
Dejean A, Lachaud J-P (2011) The hunting behavior of the African ponerine ant Pachycondyla pachyderma. Behav Process 86(2):169–173. doi:10.1016/j.beproc.2010.11.004
Domenici P (2001) The scaling of locomotor performance in predator–prey encounters: from fish to killer whales. Compar Biochem Phys A 131(1):169–182. doi:10.1016/s1095-6433(01)00465-2
Eaton RC, Emberley DS (1991) How stimulus direction determines the trajectory of the Mauthner-initiated escape response in a teleost fish. J Exp Biol 161:469–487
Estes RD (1991) The behavior guide to African mammals. University of California Press, Berkeley
Fanshawe JH, Fitzgibbon CD (1993) Factors influencing the hunting success of an African wild dog pack. Anim Behav 45(3):479–490. doi:10.1006/anbe.1993.1059
Fish FE (1993) Power output and propulsive efficiency of swimming bottle-nosed dolphins (Tursiops truncatus). J Exp Biol 185:179–193
Fréon P, Misund OA (1999) Dynamics of pelagic fish distribution and behaviour: effects on fisheries and stock assessment. Fishing News Books. Blackwell, London
Fryxell JM, Mosser A, Sinclair ARE, Packer C (2007) Group formation stabilizes predator–prey dynamics. Nature 449(7165):1041–U1044. doi:10.1038/nature06177
Gerlotto F, Bertrand S, Bez N, Gutierrez M (2006) Waves of agitation inside anchovy schools observed with multibeam sonar: a way to transmit information in response to predation. ICES J Mar Sci 63(8):1405–1417
Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, Goss-Custard J, Grand T, Heinz SK, Huse G, Huth A, Jepsen JU, Jørgensen C, Mooij WM, Müller B, Pe’er G, Piou C, Railsback SF, Robbins AM, Robbins MM, Rossmanith E, Ruger N, Strand E, Souissi S, Stillman RA, Vabø R, Visser U, DeAngelis DL (2006) A standard protocol for describing individual-based and agent-based models. Ecol Model 198(1–2):115–126
Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J, Railsback SF (2010) The ODD protocol: a review and first update. Ecol Model 221(23):2760–2768. doi:10.1016/j.ecolmodel.2010.08.019
Hamilton WD (1971) Geometry for the selfish herd. J Theor Biol 31:295–311
Handegard NO, Boswell KM, Ioannou CC, Leblanc SP, Tjostheim DB, Couzin ID (2012) The dynamics of coordinated group hunting and collective information transfer among schooling prey. Curr Biol 22(13):1213–1217. doi:10.1016/j.cub.2012.04.050
Harrison NM, Whitehouse MJ, Heinemann D, Prince PA, Hunt GL, Veit RR (1991) Observations of multispecies seabird flocks around South Georgia. Auk 108(4):801–810
Hemelrijk CK, Hildenbrandt H (2012) Schools of fish and flocks of birds: their shape and internal structure by self-organization. Interface Focus 2(6):726–737. doi:10.1098/rsfs.2012.0025
Hodges CL, Woehler EJ (1994) Associations between seabirds and cetaceans in the Australian sector of the southern Indian Ocean. Mar Ornithol 22:205–212
Hoffman W, Heinemann D, Wiens JA (1981) The ecology of seabird feeding flocks in Alaska. Auk 98(3):437–456. doi:10.2307/4086112
Huth A, Wissel C (1992) The simulation of the movement of fish schools. J Theor Biol 156:365–385
Inada Y, Kawachi K (2002) Order and flexibility in the motion of fish schools. J Theor Biol 214(3):371–387
Ioannou CC, Guttal V, Couzin ID (2012) Predatory fish select for coordinated collective motion in virtual prey. Science 337(6099):1212–1215. doi:10.1126/science.1218919
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Kunz H, Hemelrijk CK (2003) Artificial fish schools: collective effects of school size, body size, and body form. Artif Life 9(3):237–253
Kunz H, Hemelrijk CK (2012) Simulations of the social organization of large schools of fish whose perception is obstructed. Appl Anim Behav Sci 138:142–151
Lee DN, Reddish PE (1981) Plummeting gannets: a paradigm of ecological optics. Nature 293(5830):293–294. doi:10.1038/293293a0
Lee S-H, Pak HK, Chon T-S (2006) Dynamics of prey-flock escaping behavior in response to predator’s attack. J Theor Biol 240(2):250–259
Lett C, Auger P, Gaillard JM (2004) Continuous cycling of grouped vs. solitary strategy frequencies in a predator–prey model. Theor Popul Biol 65(3):263–270
Lett C, Mirabet V (2008) Modelling the dynamics of animal groups in motion. S Afr J Sci 104(5/6):192–198
Luque SP, Guinet C (2007) A maximum likelihood approach for identifying dive bouts improves accuracy, precision and objectivity. Behaviour 144:1315–1332. doi:10.1163/156853907782418213
Magurran AE, Pitcher TJ (1987) Provenance, shoal size and the sociobiology of predator-evasion behavior in minnow shoals. Proc R Soc Lond Ser B-Biol Sci 229(1257):439–465. doi:10.1098/rspb.1987.0004
Major PF (1978) Predator–prey interactions in two schooling fishes, Caranx ignobilis and Stolephorus purpureus. Anim Behav 26(AUG):760–777. doi:10.1016/0003-3472(78)90142-2
Mirabet V, Auger P, Lett C (2007) Spatial structures in simulations of animal grouping. Ecol Model 201(3–4):468–476
Moffett MW (1988) Foraging dynamics in the group-hunting myrmicine ant, Pheidologeton diversus. J Insect Behav 1(3):309–331
Muro C, Escobedo R, Spector L, Coppinger RP (2011) Wolf-pack (Canis lupus) hunting strategies emerge from simple rules in computational simulations. Behav Process 88(3):192–197. doi:10.1016/j.beproc.2011.09.006
Niizato T, Gunji Y-P (2011) Metric-topological interaction model of collective behavior. Ecol Model 222(17):3041–3049. doi:10.1016/j.ecolmodel.2011.06.008
Niizato T, Gunji Y-P (2012) Fluctuation-driven flocking movement in three dimensions and scale-free correlation. PLoS One 7(5):e35615. doi:10.1371/journal.pone.0035615
Nishimura SI, Ikegami T (1997) Emergence of collective strategies in a prey–predator game model. Artif Life 3:243–260
Nøttestad L, Axelsen BE (1999) Herring schooling manoeuvres in response to killer whale attacks. Can J Zool 77(10):1540–1546. doi:10.1139/cjz-77-10-1540
O’Donoghue SH, Drapeau L, Peddemors VM (2010) Broad-scale distribution patterns of sardine and their predators in relation to remotely sensed environmental conditions during the KwaZulu-Natal sardine run. Afr J Mar Sci 32(2):279–291. doi:10.2989/1814232x.2010.501584
Okunishi T, Yamanaka Y, Ito S (2009) A simulation model for Japanese sardine (Sardinops melanostictus) migrations in the western North Pacific. Ecol Model 220(4):462–479. doi:10.1016/j.ecolmodel.2008.10.020
Oro D (1995) Audouin’s gulls Larus audouinii associate with sub-surface predators in the Mediterranean Sea. J Ornithol 136(4):465–467. doi:10.1007/bf01651595
Packer C, Ruttan L (1988) The evolution of cooperative hunting. Am Nat 132(2):159–198. doi:10.1086/284844
Partridge BL (1982) The structure and function of fish schools. Sci Am 246(6):114–123
Pennycuick CJ (1987) Flight of auks (Alcidae) and other northern seabirds compared with southern Procellariiformes: ornitholdolite observations. J Exp Biol 128:335–347
Peraltilla S, Bertrand S (2014) In situ measurements of the speed of Peruvian anchovy schools. Fish Res 149:92–94. doi:10.1016/j.fishres.2013.09.002
Pitcher TJ, Misund OA, Fernø A, Totland B, Melle V (1996) Adaptive behaviour of herring schools in the Norwegian Sea as revealed by high-resolution sonar. ICES J Mar Sci 53(2):449–452. doi:10.1006/jmsc.1996.0063
Pitcher TJ, Wyche CJ (1983) Predator avoidance behaviour of sand-eel schools: why school seldom split? In: Noakes DLG, Lindquist BG, Helfman GS, Ward JA (eds) Predators and prey in fishes. Junk, The Hague, pp 193–204
Quérouil S, Silva MA, Cascão I, Magalhães S, Seabra MI, Machete MA, Santos RS (2008) Why do dolphins form mixed-species asssociations in the Azores? Ethology 114(12):1183–1194. doi:10.1111/j.1439-0310.2008.01570.x
Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA (2003) Spontaneous emergence of leaders and followers in foraging pairs. Nature 423(6938):432–434. doi:10.1038/nature01630
Rohr JJ, Fish FE, Gilpatrick JW (2002) Maximum swim speeds of captive and free-ranging delphinids: critical analysis of extraordinary performance. Mar Mammal Sci 18(1):1–19. doi:10.1111/j.1748-7692.2002.tb01014.x
Ropert-Coudert Y, Grémillet D, Kato A, Ryan PG, Naito Y, Le Maho Y (2004) A fine-scale time budget of Cape gannets provides insights into the foraging strategies of coastal seabirds. Anim Behav 67:985–992
Sand H, Wikenros C, Wabakken P, Liberg O (2006) Effects of hunting group size, snow depth and age on the success of wolves hunting moose. Anim Behav 72:781–789. doi:10.1016/j.anbehav.2005.11.030
Scheel D, Packer C (1991) Group hunting behavior of lions: a search for cooperation. Anim Behav 41:697–709. doi:10.1016/s0003-3472(05)80907-8
Schellinck J, White T (2011) A review of attraction and repulsion models of aggregation: methods, findings and a discussion of model validation. Ecol Model 222(11):1897–1911. doi:10.1016/j.ecolmodel.2011.03.013
Stander PE (1992) Cooperative hunting in lions: the role of the individual. Behav Ecol Sociobiol 29(6):445–454
Stanford CB (1995) The influence of chimpanzee predation on group size and antipredator behavior in red Columbus monkeys. Anim Behav 49(3):577–587. doi:10.1016/0003-3472(95)90033-0
Stensland E, Angerbjörn A, Berggren P (2003) Mixed species groups in mammals. Mammal Rev 33(3–4):205–223. doi:10.1046/j.1365-2907.2003.00022.x
Treherne JE, Foster WA (1981) Group transmission of predator avoidance behaviour in a marine insect: the Trafalgar effect. Anim Behav 29(AUG):911–917. doi:10.1016/s0003-3472(81)80028-0
Tu S-Y, Sayed AH (2011) Cooperative prey herding based on diffusion adaptation. In: 2011 I.E. International Conference on Acoustics, Speech, and Signal Processing. International Conference on Acoustics Speech and Signal Processing ICASSP. pp 3752–3755.
Vabø R, Nøttestad L (1997) An individual based model of fish school reactions: predicting antipredator behaviour as observed in nature. Fish Oceanogr 6(3):155–171
Vaughn R, Würsig B, Packard J (2010) Dolphin prey herding: prey ball mobility relative to dolphin group and prey ball sizes, multispecies associates, and feeding duration. Mar Mammal Sci 26(1):213–225. doi:10.1111/j.1748-7692.2009.00317.x
Vaughn RL, Muzi E, Richardson JL, Würsig B (2011) Dolphin bait-balling behaviors in relation to prey ball escape behaviors. Ethology 117(10):859–871. doi:10.1111/j.1439-0310.2011.01939.x
Vaughn RL, Shelton DE, Timm LL, Watson LA, Wuersig B (2007) Dusky dolphin (Lagenorhynchus obscurus) feeding tactics and multi-species associations. N Z J Mar Freshw Res 41(4):391–400
Vaughn RL, Würsig B, Shelton DS, Timm LL, Watson LA (2008) Dusky dolphins influence prey accessibility for seabirds in admiralty bay, New Zealand. J Mammal 89(4):1051–1058. doi:10.1644/07-mamm-a-145.1
Viscido SV, Miller M, Wethey DS (2001) The response of a selfish herd to an attack from outside the group perimeter. J Theor Biol 208(3):315–328. doi:10.1006/jtbi.2000.2221
Viscido SV, Parrish JK, Grünbaum D (2005) The effect of population size and number of influential neighbors on the emergent properties of fish schools. Ecol Model 183(2–3):347–363
Viscido SV, Parrish JK, Grünbaum D (2007) Factors influencing the structure and maintenance of fish schools. Ecol Model 206(1–2):153–165. doi:10.1016/j.ecolmodel.2007.03.042
Ward CR, Gobet F, Kendall G (2001) Evolving collective behavior in an artificial ecology. Artif Life 7(2):191–209. doi:10.1162/106454601753139005
Wood AJ, Ackland GJ (2007) Evolving the selfish herd: emergence of distinct aggregating strategies in an individual-based model. Proc R Soc B-Biol Sci 274(1618):1637–1642. doi:10.1098/rspb.2007.0306
Zheng M, Kashimori Y, Hoshino O, Fujita K, Kambara T (2005) Behavior pattern (innate action) of individuals in fish schools generating efficient collective evasion from predation. J Theor Biol 235(2):153–167
Acknowledgments
We thank the developers of the SwarmWatch 1.0 software (http://angel.elte.hu/starling/Demos.html) that we used to visualize our model outputs, and particularly Péter Szabó. We thank two anonymous reviewers for their very helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Note: To avoid excessive duration, all videos frame rate is set to 20 frames s−1. As the duration of the simulation timestep is 0.1 s (Table 2), 2 s in simulations correspond to 1 s in videos. To avoid excessive size, all videos are compressed using XviD MPEG-4 codec.
Video 1
Simulation S1 (Table 3) with successive predator attacks occurring every 5 s. The prey aggregation dynamics is a succession of expanding-grouping-swarming phases. (AVI 2264 kb)
Video 2
Simulation S1 with successive predator attacks occurring every 0.5 s. The prey aggregation eventually forms a quasi-permanent ring structure. (AVI 2244 kb)
Video 3
Simulation S2 with successive predator attacks occurring every 0.5 s. (AVI 2236 kb)
Video 4
Simulation S3 (including alignment in prey-prey interactions) with successive predator attacks occurring every 5 s. The prey aggregation dynamics is a succession of expanding-grouping-schooling phases. (AVI 2265 kb)
Rights and permissions
About this article
Cite this article
Lett, C., Semeria, M., Thiebault, A. et al. Effects of successive predator attacks on prey aggregations. Theor Ecol 7, 239–252 (2014). https://doi.org/10.1007/s12080-014-0213-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-014-0213-0