Abstract
Purpose
The main goals of this study were to investigate (1) the behavior of microbial communities in response to low-dose bioavailable anthracene addition in lightly contaminated sediment from Bizerte Lagoon and (2) the effects of bioremediation treatments on microbial biomass, activity, and community structure.
Methods
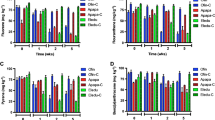
Sediment microcosms amended with 1 ppm anthracene were incubated in triplicate during 30 days. Biostimulation (addition of nitrogen and phosphorus fertilizer) and bioaugmentation (inoculation of a hydrocarbonoclastic bacterium) were used as bioremediation treatments. Bacterial biomass was estimated using flow cytometry. Sediment oxygen consumption was measured with oxygen microelectrodes. Bacterial community structure was assessed by molecular fingerprints (terminal restriction fragment length polymorphism; T-RFLP) analysis.
Results
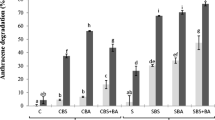
Anthracene contamination resulted in a significant reduction of bacterial abundance with an impact on cell integrity. Concomitantly, sediment oxygen consumption was strongly inhibited. Correspondence analysis on T-RFLP data indicated that bacterial community structures from anthracene-contaminated microcosms were different from that of the control. Interestingly, the changes observed in microbial biomass, structure, and activities as a result of anthracene contamination were not alleviated even with the use of biostimulation and combination of biostimulation and bioaugmentation strategy for anthracene bioremediation. Nevertheless, both treatment methods resulted in different community structures relative to the contaminated and control microcosms with the appearance of distinct populations.
Conclusion
Anthracene spiking severely affected microbial communities, suggesting dominance of nontolerant populations in this lightly-contaminated sediment. Although biostimulation and/or bioaugmentation treatments did not alleviate the anthracene toxic effects, the changes observed in microbial population and structure suggest that the proposed treatments might be promising to promote bacterial growth. Further works are still required to propose a more efficient strategy to stimulate biodegradation that takes into account the complex interactions between species for resource access.






Similar content being viewed by others
References
Atagana HI (2006) Biodegradation of polyacyclic aromatic hydrocarbons in contaminated soil by biostimulation and bioaugmentation in the presence of copper(II) ions. World J Microbiol Biotechnol 22:1145–1153
Bauer JE, Capone DG (1985) Degradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and naphthalene in intertidal marine sediments. Appl Environ Microbiol 50:81–90
Ben Said O, Goni-Urriza MS, El Bour M, Dellali M, Aissa P, Duran R (2008) Characterization of aerobic polycyclic aromatic hydrocarbon-degrading bacteria from Bizerte lagoon sediments, Tunisia. J Appl Microbiol 104:987–997
Ben Said O, Goni-Urriza M, El Bour M, Aissa P, Duran R (2010) Bacterial community structure of sediments of the Bizerte Lagoon (Tunisia), a Southern Mediterranean Coastal Anthropized Lagoon. Microb Ecol 59:445–456
Berg P, Risgaard-Petersen N, Rysgaard S (1998) Interpretation of measured concentration profiles in sediment pore water. Limnol Oceanogr 43:1500–1510
Bowling JW, Leversee GJ, Landrum PF, Giesy JP (1983) Acute mortality of anthracene-contaminated fish exposed to sunlight. Aquat Toxicol 3:79–90
Brenner RC, Magar VS, Ickes JA, Abbott JE, Stout SA, Crecelius EA, Bingler LS (2002) Characterization and FATE of PAH-contaminated sediments at the Wyckoff/Eagle Harbor superfund site. Environ Sci Technol 36:2605–2613
Bridges TS, Levin LA, Cabrera D, Plaia G (1994) Effects of sediment amended with sewage, algae, or hydrocarbons on growth and reproduction in 2 opportunistic polychaetes. J Exp Mar Biol Ecol 177:99–119
Broecker WS, Peng TH (1974) Gas exchange rates between air and sea. Tellus 26:21–35
Bugg T, Foght JM, Pickard MA, Gray MR (2000) Uptake and active efflux of polycyclic aromatic hydrocarbons by Pseudomonas fluorescens LP6a. Appl Environ Microbiol 66:5387–5392
Carman KR, Fleeger JW, Means JC, Pomarico SM, McMillin DJ (1995) Experimental investigation of the effects of polynuclear aromatic-hydrocarbons on an estuarine sediment food web. Mar Environ Res 40:289–318
Dabestani R, Ivanov IN (1999) A compilation of physical, spectroscopic and photophysical properties of polycyclic aromatic hydrocarbons. Photochem Photobiol 70:10–34
Dean-Ross D, Moody JD, Freeman JP, Doerge DR, Cerniglia CE (2001) Metabolism of anthracene by a Rhodococcus species. FEMS Microbiol Lett 204:205–211
Di Gennaro P, Moreno B, Annoni E, Garcia-Rodriguez S, Bestetti G, Benitez E (2009) Dynamic changes in bacterial community structure and in naphthalene dioxygenase expression in vermicompost-amended PAH-contaminated soils. J Hazard Mater 172:1464–1469
Duhamel S, Jacquet S (2006) Flow cytometric analysis of bacteria- and virus-like particles in lake sediments. J Microbiol Methods 64:316–332
El-Alawi YS, Dixon DG, Greenberg BM (2001) Effects of a pre-incubation period on the photoinduced toxicity of polycyclic aromatic hydrocarbons to the luminescent bacterium Vibrio fischeri. Environ Toxicol 16:277–286
El-Alawi YS, McConkey BJ, Dixon DG, Greenberg BM (2002) Measurement of short- and long-term toxicity of polycyclic aromatic hydrocarbons using luminescent bacteria. Ecotoxicol Environ Saf 51:12–21
Flores GP, Badillo CM, Cortazar MH, Hipolito CN, Perez RS, Sanchez IG (2010) Toxic effects of linear alkylbenzene sulfonate, anthracene and their mixture on growth of a microbial consortium isolated from polluted sediment. Rev Int Contam Ambient 26:39–46
Garcia HE, Gordon LI (1992) Oxygen solubility in seawater—better fitting equations. Limnol Oceanogr 37:1307–1312
Gyedu-Ababio TK, Baird D (2006) Response of meiofauna and nematode communities to increased levels of contaminants in a laboratory microcosm experiment. Ecotoxicol Environ Saf 63:443–450
Hjorth M, Vester J, Henriksen P, Forbes V, Dahllof I (2007) Functional and structural responses of marine plankton food web to pyrene contamination. Mar Ecol Prog Ser 338:21–31
Hlaili AS, Chikhaoui MA, El Grami B, Mabrouk HH (2006) Effects of n and p supply on phytoplankton in Bizerte Lagoon (western Mediterranean). J Exp Mar Biol Ecol 333:79–96
Hughes JB, Beckles DM, Chandra SD, Ward CH (1997) Utilization of bioremediation processes for the treatment of PAH-contaminated sediments. J Ind Microbiol Biotechnol 18:152–160
Jacques RJS, Okeke BC, Bento FM, Teixeira AS, Peralba MCR, Camargo FAO (2008) Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Biores Technol 99:2637–2643
James ID (2002) Modelling pollution dispersion, the ecosystem and water quality in coastal waters: a review. Environ Model Softw 17:363–385
Kochevar IE, Armstrong RB, Einbinder J, Walther RR, Harber LC (1982) Coal–tar photo-toxicity—active compounds and action spectra. Photochem Photobiol 36:65–69
Kummrow F, Rech CM, Coimbrao CA, Umbuzeiro GA (2006) Blue rayon-anchored technique/Salmonella microsome microsuspension assay as a tool to monitor for genotoxic polycyclic compounds in Santos estuary. Mutat Res Genet Toxicol Environ Mutagen 609:60–67
Lagauzere S, Pischedda L, Cuny P, Gilbert F, Stora G, Bonzom JM (2009) Influence of Chironomus riparius (Diptera, Chironomidae) and Tubifex tubifex (Annelida, Oligochaeta) on oxygen uptake by sediments. Consequences of uranium contamination. Environ Pollut 157:1234–1242
Laursen AE, Carlton RC (1999) Responses to atrazine of respiration, nitrification, and denitrification in stream sediments measured with oxygen and nitrate microelectrodes. FEMS Microbiol Ecol 29:229–240
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amesterdam
Long ER (1992) Ranges in chemical concentrations in sediments associated with adverse biological effects. Mar Pollut Bull 24:38–45
Louiz I, Kinani S, Gouze ME, Ben-Attia M, Menif D, Bouchonnet S, Porcher JM, Ben-Hassine OK, Ait-Aissa S (2008) Monitoring of dioxin-like, estrogenic and anti-androgenic activities in sediments of the Bizerta lagoon (Tunisia) by means of in vitro cell-based bioassays: Contribution of low concentrations of polynuclear aromatic hydrocarbons (PAHs). Sci Total Environ 402:318–329
Louiz I, Ben-Attia M, Ben-Hassine OK (2009) Gonadosomatic index and gonad histopathology of Gobius niger (Gobiidea, Teleost) from Bizerta lagoon (Tunisia): evidence of reproduction disturbance. Fish Res 100:266–273
Marie D, Partensky F, Jacquet S, Vaulot D (1997) Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol 63:186–193
McConkey BJ, Duxbury CL, Dixon DG, Greenberg BM (1997) Toxicity of a PAH photooxidation product to the bacteria Photobacterium phosphoreum and the duckweed Lemna gibba: effects of phenanthrene and its primary photoproduct, phenanthrenequinone. Environ Toxicol Chem 16:892–899
Miyasaka T, Asami H, Watanabe K (2006) Impacts of bioremediation schemes on bacterial population in naphthalene-contaminated marine sediments. Biodegradation 17:227–235
Muckian LM, Grant RJ, Clipson NJW, Doyle EM (2009) Bacterial community dynamics during bioremediation of phenanthrene- and fluoranthene-amended soil. Int Biodeterior Biodegrad 63:52–56
Mueller JG, Chapman PJ, Pritchard PH (1989) Creosote-contaminated sites—their potential for bioremediation. Environ Sci Technol 23:1197–1201
Myers CR, Alatalo LJ, Myers JM (1994) Microbial potential for the anaerobic degradation of simple aromatic compounds in sediments of the Milwaukee Harbor, Green Bay and Lake Erie. Environ Toxicol Chem 13:461–471
Nance JM (1991) Effects of oil gas-field produced water on the macrobenthic community in a small gradient estuary. Hydrobiologia 220:189–204
Paisse S, Goni-Urriza M, Coulon F, Duran R (2010) How a bacterial community originating from a contaminated coastal sediment responds to an oil input. Microb Ecol 60:394–405
Perelo LW (2010) Review: in situ and bioremediation of organic pollutants in aquatic sediments. J Hazard Mater 177:81–89
Pringault O, Duran R, Jacquet S, Torreton JP (2008) Temporal variations of microbial activity and diversity in marine tropical sediments (New Caledonia lagoon). Microb Ecol 55:247–258
Rasmussen H, Jorgensen BB (1992) Microelectrode studies of seasonal oxygen uptake in a coastal sediment—role of molecular diffusion. Mar Ecol Prog Ser 81:289–303
Revsbech NP (1989) An oxygen microsensor with a guard cathode. Limnol Oceanogr 34:474–478
Revsbech NP (2005) Analysis of microbial communities with electrochemical microsensors and microscale biosensors, environmental microbiology. Methods in enzymology. Elsevier, San Diego, pp 147–166
Samanta SK, Singh OV, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20:243–248
Tahir A, Fletcher TC, Houlihan DF, Secombes CJ (1993) Effect of short-term exposure to oil-contaminated sediments on the immune response of dab, Limanda limanda (L). Aquat Toxicol 27:71–82
Talley JW, Ghosh U, Tucker SG, Furey JS, Luthy RG (2002) Particle-scale understanding of the bioavailability of PAHs in sediment. Environ Sci Technol 36:477–483
Tam NFY, Ke L, Wang XH, Wong YS (2001) Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps. Environ Pollut 114:255–263
Trabelsi S, Driss MR (2005) Polycyclic aromatic hydrocarbons in superficial coastal sediments from Bizerte Lagoon, Tunisia. Mar Pollut Bull 50:344–348
Ullman WJ, Aller RC (1982) Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27:552–556
Viret H, Pringault O, Duran R (2006) Impact of zinc and nickel on oxygen consumption of benthic microbial communities assessed with microsensors. Sci Total Environ 367:302–311
Weissenfels WD, Beyer M, Klein J (1990) Degradation of phenanthrene, fluorene and fluoranthene by pure bacterial cultures. Appl Microbiol Biotechnol 32:479–484
Wunsche L, Bruggemann L, Babel W (1995) Determination of substrate utilization patterns of soil microbial communities—an approach to assess population-changes after hydrocarbon pollution. FEMS Microbiol Ecol 17:295–305
Yu KSH, Wong AHY, Yau KWY, Wong YS, Tam NFY (2005) Natural attenuation, biostimulation and bioaugmentation on biodegradation of polycyclic aromatic hydrocarbons (PAHs) in mangrove sediments. Mar Pollut Bull 51:1071–1077
Zhang SY, Wang QF, Xie SG (2011) Microbial community changes in contaminated soils in response to phenanthrene amendment. Int J Environ Sci Technol 8:321–330
Zhou HW, Wong AHY, Yu RMK, Park YD, Wong YS, Tam NFY (2009) Polycyclic aromatic hydrocarbon-induced structural shift of bacterial communities in mangrove sediment. Microb Ecol 58:153–160
Acknowledgments
This work was supported by a funding of the CMCU (n° 09G 0189) and PHC-Utique (20894TE) programs, the Centre National de la Recherche Scientifique (CNRS/DGRST n°22629), and the Ministère Tunisien de la Recherche scientifique.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor Philippe Garrigues
Rights and permissions
About this article
Cite this article
Louati, H., Said, O.B., Got, P. et al. Microbial community responses to bioremediation treatments for the mitigation of low-dose anthracene in marine coastal sediments of Bizerte lagoon (Tunisia). Environ Sci Pollut Res 20, 300–310 (2013). https://doi.org/10.1007/s11356-012-0860-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0860-x