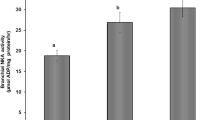
Abstract
Dicentrarchus labrax migrates between sea (SW), brackish and fresh water (FW) where chloride concentrations and requirements for chloride handling change: in FW, fish absorb chloride and restrict renal losses; in SW, they excrete chloride. In this study, the expression and localization of ClC-3 and Na+/K+-ATPase (NKA) were studied in fish adapted to SW, or exposed to FW from 10 min to 30 days. In gills, NKA-α1 subunit expression transiently increased from 10 min and reached a stabilized intermediate expression level after 24 h in FW. ClC-3 co-localized with NKA in the basolateral membrane of mitochondria-rich cells (MRCs) at all conditions. The intensity of MRC ClC-3 immunostaining was significantly higher (by 50 %) 1 h after the transfer to FW, whereas the branchial ClC-3 protein expression was 30 % higher 7 days after the transfer as compared to SW. This is consistent with the increased number of immunopositive MRCs (immunostained for NKA and ClC-3). However, the ClC-3 mRNA expression was significantly lower in FW gills. In the kidney, after FW transfer, a transient decrease in NKA-α1 subunit expression was followed by significantly higher stable levels from 24 h. The low ClC-3 protein expression detected at both salinities was not observed by immunocytochemistry in the SW kidney; ClC-3 was localized in the basal membrane of the collecting ducts and tubules 7 and 30 days after transfer to FW. Renal ClC-3 mRNA expression, however, seemed higher in SW than in FW. The potential role of this chloride channel ClC-3 in osmoregulatory and osmosensing mechanisms is discussed.









Similar content being viewed by others
References
Alvarado NE, Quesada I, Hylland K, Marigomez I, Soto M (2006) Quantitative changes in metallothionein expression in target cell-types in the gills of turbot (Scophthalmus maximus) exposed to Cd, Cu, Zn and after a depuration treatment. Aquat Toxicol 77:64–77
Bennetts B, Rychkov GY, Ng H-L, Morton CJ, Stapleton D, Parker MW, Cromer BA (2005) Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J Biol Chem 280(37):32452–32458. doi:10.1074/jbc.M502890200
Bodinier C, Boulo V, Lorin-Nebel C, Charmantier G (2009a) Influence of salinity on the localization and expression of the CFTR chloride channel in the ionocytes of Dicentrarchus labrax during ontogeny. J Anat 214(3):318–329
Bodinier C, Lorin-Nebel C, Charmantier G, Boulo V (2009b) Influence of salinity on the localization and expression of the CFTR chloride channel in the ionocytes of juvenile Dicentrarchus labrax exposed to seawater and freshwater. Comp Biochem Physiol A 153:345–351
Bodinier C, Lorin-Nebel C, Charmantier G, Boulo V (2009c) Influence of salinity on the localization and expression of the CFTR chloride channel in the ionocytes of juvenile Dicentrarchus labrax exposed to seawater and freshwater. Comp Biochem Physiol A 153(3):345–351
Bollag DM, Rozycki MD, Edelstein SJ (1996) Gel electrophoresis under denaturing conditions. In: Protein methods. Wiley-Liss, New York, p 415
Bossus M, Charmantier G, Lorin-Nebel C (2011) Transient receptor potential vanilloid 4 in the European sea bass Dicentrarchus labrax: a candidate protein for osmosensing. Comp Biochem Physiol A 160(1):43–51. doi:10.1016/j.cbpa.2011.04.014
Boutet I, Long Ky CL, Bonhomme F (2006) A transcriptomic approach of salinity response in the euryhaline teleost, Dicentrarchus labrax. Gene 379:40–50
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Braithwaite VA (2011) Fish learning and memory. In: Farrell AP (ed) Encyclopedia of fish physiology: from genome to environment, vol 1. Elsevier, Vancouver, pp 707–712
Bystriansky JS, Richards JG, Schulte PM, Ballantyne JS (2006) Reciprocal expression of gill Na+/K+-ATPase α-subunit isoforms α1a and α1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J Exp Biol 209(10):1848–1858. doi:10.1242/jeb.02188
Carr G, Simmons N, Sayer J (2003) A role for CBS domain 2 in trafficking of chloride channel CLC-5. Biochem Biophys Res Commun 310(2):600–605. doi:10.1016/j.bbrc.2003.09.057
Chang W, Loretz CA (1993) DPC blockade of transepithelial chloride absorption and single anion channels in teleost urinary bladder. Am J Physiol—Regulat Integr Comp Physiol 265(1):R66–R75
Chen G, Gharib TG, Huang C–C, Taylor JMG, Misek DE, Kardia SLR, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 1(4):304–313. doi:10.1074/mcp.M200008-MCP200
Choi JH, Lee KM, Inokuchi M, Kaneko T (2011) Morphofunctional modifications in gill mitochondria-rich cells of Mozambique tilapia transferred from freshwater to 70 % seawater, detected by dual observations of whole-mount immunocytochemistry and scanning electron microscopy. Comp Biochem Physiol A 158(1):132–142. doi:10.1016/j.cbpa.2010.09.019
Chu S, Zeitlin PL (1997) Alternative mRNA splice variants of the rat ClC-2 chloride channel gene are expressed in lung: genomic sequence and organization of ClC-2. Nucleic Acids Res 25(20):4153–4159. doi:10.1093/nar/25.20.4153
Coca-Prados M, Sánchez-Torres J, Peterson-Yantorno K, Civan MM (1996) Association of ClC-3 channel with Cl− transport by human nonpigmented ciliary epithelial cells. J Membr Biol 150(2):197–208. doi:10.1007/s002329900044
Dave S, Sheehan J, Meiler J, Strange K (2010) Unique gating properties of C. elegans ClC anion channel splice variants are determined by altered CBS domain conformation and the R-helix linker. Channels 4(4):289–301
Decker CJ, Parker R (1994) Mechanisms of mRNA degradation in eukaryotes. Trends Biochem Sci 19(8):336–340. doi:10.1016/0968-0004(94)90073-6
Do CW, Lu W, Mitchell CH, Civan MM (2005) Inhibition of swelling-activated Cl− currents by functional anti-ClC-3 antibody in native bovine non-pigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci 46(3):948–955. doi:10.1167/iovs.04-1004
Duan D, Winter C, Cowley S, Hume JR, Horowitz B (1997) Molecular identification of a volume-regulated chloride channel. Nature 390(6658):417–421
Duan D, Cowley S, Horowitz B, Hume JR (1999) A serine residue in ClC-3 links phosphorylation–dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol 113(1):57–70. doi:10.1085/jgp.113.1.57
Duan D, Zhong J, Hermoso M, Satterwhite CM, Rossow CF, Hatton WJ, Yamboliev I, Horowitz B, Hume JR (2001) Functional inhibition of native volume-sensitive outwardly rectifying anion channels in muscle cells and Xenopus oocytes by anti-ClC-3 antibody. J Physiol 531(2):437–444. doi:10.1111/j.1469-7793.2001.0437i.x
Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415:287–294
Eggermont J, Buyse G, Voets T, Tytgat J, De Smedt H, Droogmans G, Nilius B (1997) Alternative splicing of ClC-6 (a member of the CIC chloride-channel family) transcripts generates three truncated isoforms one of which, ClC-6c, is kidney-specific. Biochem J 325:269–276
Estévez R, Jentsch TJ (2002) CLC chloride channels: correlating structure with function. Curr Opin Struct Biol 12(4):531–539. doi:10.1016/s0959-440x(02)00358-5
Estévez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ (2004) Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol 557(2):363–378. doi:10.1113/jphysiol.2003.058453
Evans DH (2009) Osmotic and ionic regulation. Cells and animals, vol 1. CRC Press, Boca Raton
Evans DH, Claiborne JB (2006) The physiology of fishes, 3rd edn. CRC Press, Boca Raton
Evans DH, Piermarini PM, Potts WTW (1999) Ionic transport in the fish gill epithelium. J Exp Zool 283:641–652
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85(1):97–177. doi:10.1152/physrev.00050.2003
Fahlke C, Yu HT, Beck CL, Rhodes TH, George AL (1997) Pore-forming segments in voltage-gated chloride channels. Nature 390(6659):529–532
Frost P, Nilsen F (2003) Validation of reference genes for transcription profiling in the salmon louse, Lepeaphtheirus salmonis, by quantitative real-time PCR. Vet Parasitol 118:169–174
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Giffard-Mena I, Charmantier G, Grousset E, Aujoulat F, Castille R (2006) Digestive tract ontogeny of Dicentrarchus labrax: implication in osmoregulation. Dev Growth Differ 48:139–151
Giffard-Mena I, Lorin-Nebel C, Charmantier G, Castille R, Boulo V (2008) Adaptation of the sea-bass (Dicentrarchus labrax) to fresh water: role of aquaporins and Na+/K+-ATPases. Comp Biochem Physiol A 150(3):332–338. doi:10.1016/j.cbpa.2008.04.004
Gonzalez RJ (2011) The osmorespiratory compromise. In: Farrell AP (ed) Encyclopedia of fish physiology: from genome to environment, vol 2. Gas exchange, internal homeostatis, and food uptake. Elsevier, Amsterdam, pp 1389–1394
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4(9):117
Gupta R, Brunak S (2002) Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput 7:310–322
He L, Denton J, Nehrke K, Strange K (2006) Carboxy terminus splice variation alters ClC channel gating and extracellular cysteine reactivity. Biophys J 90(10):3570–3581
Hebeisen S, Biela A, Giese B, Müller-Newen G, Hidalgo P, Fahlke C (2004) The role of the carboxyl terminus in ClC chloride channel function. J Biol Chem 279(13):13140–13147. doi:10.1074/jbc.M312649200
Hermoso M, Satterwhite CM, Andrade YN, Hidalgo J, Wilson SM, Horowitz B, Hume JR (2002) ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl− channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J Biol Chem 277(42):40066–40074. doi:10.1074/jbc.M205132200
Hickman CP, Trump BF (1969) The kidney. In: Hoar WS, Randall DJ (eds) Fish physiology, vol I. Academic Press, New York, pp 91–239
Hiroi J, Kaneko T, Tanaka M (1999) In vivo sequential changes in chloride cell morphology in the yolk-sac membrane of mozambique tilapia (Oreochromis mossambicus) embryos and larvae during seawater adaptation. J Exp Biol 202(24):3485–3495
Hiroi J, Yasumasu S, McCormick S, Hwang PP, Kaneko T (2008) Evidence for an apical Na–Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211:2584–2599
Hirose S, Kaneko T, Naito N, Takei Y (2003) Molecular biology of major components of chloride cells. Comp Biochem Physiol B 136(4):593–620. doi:10.1016/s1096-4959(03)00287-2
Hoffmann EK, Schettino T, Marshall WS (2007) The role of volume-sensitive ion transport systems in regulation of epithelial transport. Comp Biochem Physiol A 148(1):29–43. doi:10.1016/j.cbpa.2006.11.023
Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ (2001) Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem 276(23):20093–20100. doi:10.1074/jbc.M009376200
Hwang P–P, Lee T-H (2007) New insights into fish ion regulation and mitochondrion-rich cells. Comp Biochem Physiol A 148(3):479–497. doi:10.1016/j.cbpa.2007.06.416
Hwang PP, Lee TH, Lin LY (2011) Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301(1):R28–R47. doi:10.1152/ajpregu.00047.2011
Ignoul S, Eggermont J (2005) CBS domains: structure, function and pathology in human proteins. Am J Physiol Cell Physiol 289:1369–1378
Inokuchi M, Hiroi J, Watanabe S, Lee KM, Kaneko T (2008) Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp Biochem Physiol A 151(2):151–158
Inokuchi M, Hiroi J, Watanabe S, Hwang P–P, Kaneko T (2009) Morphological and functional classification of ion-absorbing mitochondria-rich cells in the gills of Mozambique tilapia. J Exp Biol 212(7):1003–1010. doi:10.1242/jeb.025957
Jensen MK, Madsen SS, Kristiansen K (1998) Osmoregulation and salinity effects on the expression and activity of Na+, K+-ATPase in the gills of European sea bass, Dicentrarchus labrax (L.). J Exp Zool 282(3):290–300
Jentsch TJ (2008) CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43(1):3–36. doi:10.1080/10409230701829110
Jentsch TJ, Steinmeyer K, Schwarz G (1990) Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature 348(6301):510–514
Jentsch TJ, Gunther W, Pusch M, Schwappach B (1995) Properties of voltage-gated chloride channels of the ClC gene family. J Physiol 482:19S–25S
Jentsch TJ, Friedrich T, Schriever A, Yamada H (1999) The CLC chloride channel family. Pflügers Arch 437(6):783–795. doi:10.1007/s004240050847
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82(2):503–568. doi:10.1152/physrev.00029.2001
Jentsch TJ, Poet M, Fuhrmann JC, Zdebik AA (2005) Physiological functions of ClC Cl− channels gleaned from human genetic disease and mouse models. Annu Rev Physiol 67:779–807
Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S (1994) Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron 12(3):597–604. doi:10.1016/0896-6273(94)90215-1
Kemp BE (2004) Bateman domains and adenosine derivatives form a binding contract. J Clin Invest 113(2):182–184
Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ (1994) Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci 91(15):6943–6947
Kushwaha H, Singh A, Sopory S, Singla-Pareek S, Pareek A (2009) Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics 10(1):200
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Lasserre P (1971) Increase of (Na+/K+)-dependent ATPase activity in gills and kidneys of two euryhaline marine teleosts, Crenimugil labrosus (Risso, 1826) and Dicentrarchus labrax (Linnaeus, 1758), during adaptation to fresh water. Life Sci 10:113–119
Lin CH, Tsai RS, Lee TH (2004) Expression and distribution of Na, K-ATPase in gill and kidney of the spotted green pufferfish, Tetraodon nigroviridis, in response to salinity challenge. Comp Biochem Physiol A 138(3):287–295. doi:10.1016/j.cbpb.2004.04.005
Lin YM, Chen CN, Yoshinaga T, Tsai SC, Shen ID, Lee TH (2006) Short-term effects of hyposmotic shock on Na+/K+-ATPase expression in gills of the euryhaline milkfish, Chanos chanos. Comp Biochem Physiol A 143(3):406–415. doi:10.1016/j.cbpa.2005.12.031
Loewen ME, MacDonald DW, Gaspar KJ, Forsyth GW (2000) Isoform-specific exon skipping in a variant form of ClC-2. Biochimica et Biophysica Acta (BBA)—Gene Struct Expr 1493(1–2):284–288. doi:10.1016/s0167-4781(00)00181-0
Lorin-Nebel C, Boulo V, Bodinier C, Charmantier G (2006) The Na+/K+/2Cl− cotransporter in the sea bass Dicentrarchus labrax during ontogeny: involvement in osmoregulation. J Exp Biol 209(24):4908–4922. doi:10.1242/jeb.02591
Marshall WS (2002) Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis. J Exp Zool 293(3):264–283. doi:10.1002/jez.10127
Marshall WS, Grosell M (2005) Ion transport, osmoregulation, and acid–base balance. In: Evans DH, Claiborne J (eds) The physiology of fishes. CRC Press, Boca Raton, pp 177–230
Marshall WS, Lynch EM, Cozzi RRF (2002) Redistribution of immunofluorescence of CFTR anion channel and NKCC cotransporter in chloride cells during adaptation of the killifish Fundulus heteroclitus to sea water. J Exp Biol 205(9):1265–1273
McCormick SD (2011) The hormonal control of osmoregulation in teleost fish. In: Farrell AP (ed) Encyclopedia of fish physiology: from genome to environment, vol 2., 1 ednAcademic Press, San Diego, pp 1466–1474
McCormick SD, Regish AM, Christensen AK (2009) Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J Exp Biol 212(24):3994–4001. doi:10.1242/jeb.037275
Meier M, Janosik M, Kery V, Kraus JP, Burkhard P (2001) Structure of human cystathionine β-synthase: a unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J 20(15):3910–3916
Mindell JA, Maduke M (2001) ClC chloride channels. Genome Biol 2(2):3000.3001–3000.3006
Miyazaki H, Uchida S, Takei Y, Hirano T, Marumo F, Sasaki S (1999) Molecular cloning of CLC Chloride Channels in Oreochromis Mossambicus and their functional complementation of yeast CLC gene mutant. Biochem Biophys Res Commun 255(1):175–181
Miyazaki H, Kaneko T, Uchida S, Sasaki S, Takei Y (2002) Kidney-specific chloride channel, OmClC-K, predominantly expressed in the diluting segment of freshwater-adapted tilapia kidney. Proc Natl Acad Sci USA 99(24):15782–15787. doi:10.1073/pnas.242611099
Nebel C, Nègre-Sadargues G, Blasco C, Charmantier G (2005a) Morphofunctional ontogeny of the urinary system of the European sea bass Dicentrarchus labrax. Anat Embryol 209(3):193–206. doi:10.1007/s00429-004-0438-6
Nebel C, Romestand B, Nègre-Sadargues G, Grousset E, Aujoulat F, Bacal J, Bonhomme F, Charmantier G (2005b) Differential freshwater adaptation in juvenile sea-bass Dicentrarchus labrax: involvement of gills and urinary system. J Exp Biol 208:3859–3871
Nehrke K, Begenisich T, Pilato J, Melvin JE (2000) Into ion channel and transporter function. Caenorhabditis elegans ClC-type chloride channels: novel variants and functional expression. Am J Physiol Cell Physiol 279(6):C2052–C2066
Nilsson GE (2011) Gas exchange. In: AP Farrell (ed) Encyclopedia of fish physiology: from genome to environment, vol 2. Gas exchange, internal homeostatis, and food uptake. Elsevier, Amsterdam, pp 796–802
Nishimura H, Imai M (1982) Control of renal function in freshwater and marine teleosts. Fed Proc 41:2355–2360
Rankin JC, Davenport JA (1981) Movement between fresh water and sea water. In: Rankin JC, Davenport JA (eds) Animal osmoregulation. Blackie, Glasgow, pp 83–100
Richards JG, Semple JW, Bystriansky JS, Schulte PM (2003) Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J Exp Biol 206(24):4475–4486. doi:10.1242/jeb.00701
Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ (2004) Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol 556(2):353–368. doi:10.1113/jphysiol.2003.058032
Rutledge E, Denton J, Strange K (2002) Cell cycle- and swelling-induced activation of a Caenorhabditis elegans ClC channel is mediated by CeGLC-7α/β phosphatases. J Cell Biol 158(3):435–444. doi:10.1083/jcb.200204142
Sardella BA, Matey V, Cooper J, Gonzalez RJ, Brauner CJ (2004) Physiological, biochemical and morphological indicators of osmoregulatory stress in ‘California’ Mozambique tilapia (Oreochromis mossambicus × O. urolepis hornorum) exposed to hypersaline water. J Exp Biol 207(8):1399–1413. doi:10.1242/jeb.00895
Schmidt-Rose T, Jentsch TJ (1997a) Reconstitution of functional voltage gated chloride channels from complementary fragments of ClC-1. J Biol Chem 272:20515–20521
Schmidt-Rose T, Jentsch TJ (1997b) Transmembrane topology of a CLC chloride channel. Proc Natl Acat Sci 94(14):7633–7638
Schmieder S, Lindenthal S, Ehrenfeld J (2001) Tissue-specific N-glycosylation of the ClC-3 chloride channel. Biochem Biophys Res Commun 286(3):635–640. doi:10.1006/bbrc.2001.5407
Scott GR, Richards JG, Forbush B, Isenring P, Schulte PM (2004a) Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am J Physiol Cell Physiol 287:300–309
Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG (2004b) CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113:274–284
Seale AP, Watanabe S, Breves JP, Lerner DT, Kaneko T, Gordon Grau E (2012) Differential regulation of TRPV4 mRNA levels by acclimation salinity and extracellular osmolality in euryhaline tilapia. Gen Comp Endocrinol 178(1):123–130. doi:10.1016/j.ygcen.2012.04.020
Shen ID, Chiu YH, Lee TH, Hwang PP (2007) Localization of chloride transporters in gill epithelia of the grass pufferfish, Takifugu niphobles. J Fish Soc Taiwan 34(1):87–100
Shimada K, Li X, Xu G, Nowak DE, Showalter LA, Weinman SA (2000) Expression and canalicular localization of two isoforms of the ClC-3 chloride channel from rat hepatocytes. Am J Physiol 279(2):G268–G276
Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29:185–196
Sucré E, Bossus M, Bodinier C, Boulo V, Charmantier G, Charmantier-Daures M, Cucchi-Mouillot P (2012) Osmoregulatory response to low salinities in the European sea bass embryos: a multi site approach. J Comp Physiol B (in press)
Takeyasu K, Tamkun MM, Renaud KJ, Fambrough DM (1988) Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem 263(9):4347–4354
Tang CH, Lee TH (2007) The effect of environmental salinity on the protein expression of Na+/K+-ATPase, Na+/K+/2Cl− cotransporter, cystic fibrosis transmembrane conductance regulator, anion exchanger 1, and chloride channel 3 in gills of a euryhaline teleost, Tetraodon nigroviridis. Comp Biochem Physiol A 147(2):521–528
Tang CH, Lee TH (2011) Ion-deficient environment induces the expression of basolateral chloride channel, ClC-3-like protein, in gill mitochondrion-rich cells for chloride uptake of the Tilapia Oreochromis mossambicus. Physiol Biochem Zool 84(1):54–67. doi:10.1086/657161
Tang CH, Hwang LY, Lee TH (2010) Chloride channel ClC-3 in gills of the euryhaline teleost, Tetraodon nigroviridis: expression, localization and the possible role of chloride absorption. J Exp Biol 213(5):683–693. doi:10.1242/jeb.040212
Tang CH, Hwang LY, Shen ID, Chiu YH, Lee TH (2011) Immunolocalization of chloride transporters to gill epithelia of euryhaline teleosts with opposite salinity-induced Na+/K+-ATPase responses. Fish Physiol Biochem 37(4):709–724. doi:10.1007/s10695-011-9471-6
Taylor ME, Drickamer K (2003) Introduction to glycobiology, 1st edn. Oxford University Press, Oxford
Vanoye CG, George AL (2002) Functional characterization of recombinant human ClC-4 chloride channels in cultured mammalian cells. J Physiol 539(2):373–383. doi:10.1113/jphysiol.2001.013115
Vargas JP, López JC, Portavella M (2009) What are the functions of fish brain pallium? Brain Res Bull 79(6):436–440. doi:10.1016/j.brainresbull.2009.05.008
Varsamos S, Diaz JP, Charmantier G, Flik G, Blasco C, Connes R (2002) Branchial chloride cells in sea bass (Dicentrarchus labrax) adapted to fresh water, seawater, and doubly concentrated seawater. J Exp Biol 293:12–26
Varsamos S, Wendelaar Bonga SE, Charmantier G, Flik G (2004) Drinking and Na+/K+ ATPase activity during early development of European sea bass, Dicentrarchus labrax ontogeny and short-term regulation following acute salinity changes. J Exp Marine Biol Ecol 311:189–200
Varsamos S, Nebel C, Charmantier G (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A 141(4):401–429
Varsamos S, Xuereb B, Commes T, Flik G, Spanings-Pierrot C (2006) Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J Endocrinol 191(2):473–480. doi:10.1677/joe.1.06847
Vessey JP, Shi C, Jollimore CA, Stevens KT, Coca-Prados M, Barnes S, Kelly ME (2004) Hyposmotic activation of ICl, swell in rabbit nonpigmented ciliary epithelial cells involves increased ClC-3 trafficking to the plasma membrane. Biochem Cell Biol 82(6):708–718. doi:10.1139/o04-107
Waldegger S, Jentsch TJ (2000) From tonus to tonicity: physiology of CLC chloride channels. J Am Soc Nephr 11(7):1331–1339
Wang L, Chen L, Jacob TJC (2000) The role of ClC-3 in volume-activated chloride currents and volume regulation in bovine epithelial cells demonstrated by antisense inhibition. J Physiol 524(1):63–75. doi:10.1111/j.1469-7793.2000.t01-1-00063.x
Wang G-X, Hatton WJ, Wang GL, Zhong J, Yamboliev I, Duan D, Hume JR (2003) Functional effects of novel anti-ClC-3 antibodies on native volume-sensitive osmolyte and anion channels in cardiac and smooth muscle cells. Am J Physiol Heart Circ Physiol 285(4):H1453–H1463. doi:10.1152/ajpheart.00244.2003
Wilson JM, Laurent P (2002) Fish gill morphology: inside out. J Exp Zool 293(3):192–213. doi:10.1002/jez.10124
Wilson JM, Laurent P, Tufts BL, Benos DJ, Donowitz M, Vogl AW, Randall DJ (2000a) NaCl uptake by the branchial epithelium in freshwater teleost fish: an immunological approach to ion-transport protein localization. J Exp Biol 203(15):2279–2296
Wilson JM, Randall DJ, Donowitz M, Vogl AW, Ip AK (2000b) Immunolocalization of ion-transport proteins to branchial epithelium mitochondria-rich cells in the mudskipper (Periophthalmodon schlosseri). J Exp Biol 203(15):2297–2310
Acknowledgments
We wish to thank the Creufop at the Marine Biological Station of Sète for providing sea bass juveniles and Evelyse Grousset for technical help. We thank Julien Issartel and Erick Desmarais for their judicious advices, and also anonymous reviewers for their contributions to the improvement of the manuscript. Data used in this work were produced through the technical facilities of the Centre Méditerranée Environnement Biodiversité and the platform qPCR Haut Débit of the IFR122. Pictures of immunostaining were produced through the Montpellier RIO Imaging platform. Maryline Bossus thanks the French government—Ministry of Research and Technology—for her ATP (“Action Thématique Prioritaire”) Doctorate scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Bossus, M., Charmantier, G., Blondeau-Bidet, E. et al. The ClC-3 chloride channel and osmoregulation in the European Sea Bass, Dicentrarchus labrax . J Comp Physiol B 183, 641–662 (2013). https://doi.org/10.1007/s00360-012-0737-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0737-9