Abstract
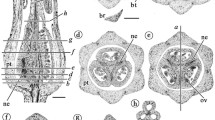
In this paper we study merosity in the genus Urospatha within the framework of a resolved phylogeny of the Araceae. We analyse how a transition from dimerous or tetramerous merosity to pentamerous or hexamerous merosity can occur developmentally in the Lasioideae. In Urospatha, initiation of floral primordia along the inflorescence is acropetal, while development of flowers is basipetal. This indicates the presence of two distinct phases in the development of the Urospatha inflorescence. The first phase corresponds to initiation of flowers and establishment of the phyllotactic pattern, and the second phase to differentiation of floral organs. Urospatha is characterized by the presence of trimerous, tetramerous, pentamerous and rarely hexamerous flowers. In all types of flowers, the stamens are closely associated and opposite to the tepals. Pentamerous flowers are formed by addition of a sector comprising a stamen and tepal. Likewise, in the case of hexamerous flowers, two sectors are added. In the Lasioideae, the increase in the number of tepals and stamens is linked with two developmental processes that have appeared independently in the subfamily: (1) addition of one or two stamen–petal sectors (Anaphyllopsis and Urospatha), and (2) independent increase in the number of tepals and stamens on whorls, more or less organized and inserted in alternate position (Dracontium). Tetramerous whorls as they occur in basal Lasioideae would be homologous to two dimerous whorls from an evolutionary point of view.





Similar content being viewed by others
References
Barabé D, Bertrand C (1996) Organogénie florale des genres Culcasia et Cercestis (Araceae). Can J Bot 74:898–908
Barabé D, Labrecque M (1983) Vascularisation de la fleur de Calla palustris (Araceae). Can J Bot 61:1718–1726
Barabé D, Lacroix C (2001) The developmental floral morphology of Montrichardia arborescens (Araceae) revisited. Bot J Linn Soc 135:413–420
Barabé D, Lacroix C (2002) Aspects of floral development in Caladium bicolor (Araceae). Can J Bot 80:899–905
Barabé D, Lacroix C (2008a) Developmental morphology of the flower of Anthurium jenmanii: a new element in our understanding of basal Araceae. Botany 86:45–52
Barabé D, Lacroix C (2008b) Developmental morphology of the flower of Anaphyllopsis americana and its relevance to our understanding of basal Araceae. Botany 86:1467–1473
Barabé D, Lacroix C (2008c) Hierarchical developmental morphology: the case of the inflorescence of Philodendron ornatum (Araceae). Int J Plant Sci 169:1013–1022
Barabé D, Bruneau A, Forest F, Lacroix C (2002) The correlation between development of atypical bisexual flowers and phylogeny in the Aroideae (Araceae). Plant Syst Evol 232:1–19
Barabé D, Lacroix C, Jeune B (2004a) The game of numbers in homeotic flowers of Philodendron (Araceae). Can J Bot 82:1459–1467
Barabé D, Lacroix C, Bruneau A, Archambault A, Gibernau M (2004b) Floral development and phylogenetic position of Schismatoglottis (Araceae). Int J Plant Sci 165:173–189
Barabé D, Lacroix C, Jeune B (2008) Quantitative developmental analysis of homeotic changes in the inflorescence of Philodendron (Araceae). Ann Bot 101:1027–1034
Barahona Carvajal ME (1977) Estudio morfologico comparativo de las inflorescencias de dos especies de Araceae: Anthurium denudatum Engler y Philodendron radiatum Schot. Rev Biol Trop 25:301–333
Bowman JL, Brüggemann H, Lee J-Y, Mummenhoff K (1999) Evolutionary changes in floral structure within Lepidium L. (Brassicaceae). Int J Plant Sci 160:917–929
Buzgo M (1994) Inflorescence development of Pistia stratiotes (Araceae). Bot Jahr 115:557–570
Buzgo M (1999) Flower structure and development of Acoraceae and basal Araceae and their systematic position among basal monocotyledons. Unpub Thesis, Zurich University (Universität Zürich), Switzerland
Buzgo M (2001) Flower structure and development of Araceae compared with alismatids and Acoraceae. Bot J Linn Soc 136:393–425
Cabrera LI, Salazar GA, Chase MW, Mayo SJ, Bogner J, Dávila P (2008) Phylogenetic relationships of Aroids and Duckweeds (Araceae) inferred from coding and non-coding plastid DNA. Am J Bot 95:1153–1165
Carvell WN (1989) Floral anatomy of the Pothoideae and Monsteroideae. (Araceae). Unpub Thesis, Department of Botany, Miami University, Oxford
Cusimano N, Bogner J, Mayo SJ, Boyce PC, Wong SY, Hesse M, Hetterscheid WLA, Keating R, French JC (2011) Relationships within the Araceae: comparison of morphological patterns with molecular phylogenies. Am J Bot 98 (in press)
Engler A, Krause K (1912) Araceae-Philodendroideae-Philodendreae. In: Engler A (ed.) Das Pflanzenreich. Regni vegetabilis conspectus, IV. 23 Da. Heft 55. Engelmann, Leipzig Reprinted 1966 (J. Cramer) pp 1–134
Eyde RH, Nicolson DH, Sherwin P (1967) A survey of floral anatomy in Araceae. Am J Bot 54:478–479
French JC (1985a) Patterns of endothecial wall thickenings in Araceae: subfamilies subfamilies Pothoideae and Monsteroideae. Am J Bot 72:472–486
French JC (1985b) Patterns of endothecial wall thickenings in Araceae: subfamilies Calloideae, Lasioideae, and Philodendroideae. Bot Gaz 146:521–533
Hay A (1992) Tribal and subtribal delimitation and circumscription of the genera of Araceae tribe Lasieae. Ann Missouri Bot Gard 79:184–205
Hotta M (1971) Study of the family Araceae: general remarks. Jpn J Bot 20:269–310
Igersheim A, Buzgo M, Endress PK (2001) Gynoecium diversity and systematics in basal monocots. Bot J Linn Soc 136:1–65
Kubitzki K (1987) Origin and significance of trimerous flowers. Taxon 36:21–28
Lehmann N, Sattler R (1992) Irregular floral development in Calla palustris (Araceae) and the concept of homeosis. Am J Bot 79:1145–1157
Mayo SJ (1986) Systematics of Philodendron Schott (Araceae) with special reference to inflorescence characters. Unpub Thesis, Department of Botany, University of Reading, UK
Mayo SJ (1989) Observations of gynoecial structure in Philodendron (Araceae). Bot J Linn Soc 100:139–172
Mayo SJ, Bogner J, Boyce PC (1997) The genera of Araceae. Royal Botanic Gardens, Kew
Poisson G, Barabé D (2011) Developmental morphology of the flower of Dracontium polyphyllum in the context of the phylogeny of the Araceae. Kew Bull (in press)
Radford AE, Dickinson WC, Masset JR, Bell CR (1974) Vascular plants systematic. Harper & Row, New York
Ronse De Craene LP, Smets EF (1994) Merosity in flowers: definition, origin, and taxonomic significance. Plant Syst Evol 191:83–104
Ronse De Craene LP (2003) The evolutionary significance of homeosis in flowers: a morphological perspective. Int J Plant Sci 164(5 Suppl):S225–S235
Sattler R (1994) Homology, homeosis and process morphology in plants. In: Hall BK (ed) Homology: the hierarchical basis of comparative biology. Academic Press, London, pp 423–475
Tucker SC (2000) Floral development and homeosis in Saraca (Leguminoseae: Caesalpinioideae: Detarieae). Int J Plant Sci 161:537–549
Uhlarz H (1982) Typologische und ontogenetische Untersuchungen an Spathicarpa sagittifolia Schott (Araceae): Wuchsform und Infloreszenz. Beit Biol Pflanz 57:389–429
Uhlarz H (1986) Zum Problem des “blattlosen Sprosses”: Morphologie und Anatomie der Infloreszenz von Pinella tripartita (Blume) Schott (Araceae, Aroideae). Beit Biol Pflanz 61:241–282
Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano H-Y (2004) The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16:500–509
Acknowledgments
This research was supported in part by operating grants from the Natural Sciences and Engineering Research Council of Canada to D.B. and C.L., and by the project “2ID” from the CNRS Amazonie Program (Conseil national de la recherche scientifique, France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barabé, D., Lacroix, C. & Gibernau, M. Floral development of Urospatha: merosity and phylogeny in the Lasioideae (Araceae). Plant Syst Evol 296, 41–50 (2011). https://doi.org/10.1007/s00606-011-0475-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0475-6