Abstract
Key message
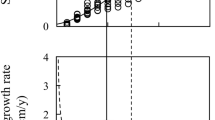
Sapwood area and the radial growth rate of the trunk follow the same pattern at breast height, with an initial increase and subsequent constant value, resulting from the increasing growth allocation toward the crown rather than tree decline. Heartwood area and heartwood volume in the trunk increase more rapidly after this shift occurs.
Abstract
Sapwood (SW) and heartwood (HW) are two functionally distinct classifications of wood in perennial stems for which quantities can vary greatly in tropical trees. Numerous positive correlations have been found between the radial growth rate (RGR) and SW quantity; however, variations in the SW/HW quantities have not been studied in light of the ontogenetic variation of RGR. Wood core sampling, intensive measurements of tree structure (number of branches, stem volumes), and radial growth monitoring were performed on an abundant and highly exploited tree species in French Guiana (Dicorynia guianensis) to investigate the relationship between RGR, SW/HW quantity, tree structure, and their variations on the course of a tree’s ontogeny. SW area and RGR followed the same pattern of variation throughout tree development, both increasing first and reaching a steady state after 50 cm DBH (diameter at breast height). After this value, we observed a strong increase in both the HW area and HW volume increment, concomitant with a more rapid increase in crown volume. The stabilization of RGR for trees with DBH > 50 cm was related not to a tree’s decline but rather to an increasing wood allocation to the crown, confirming that RGR at breast height is a poor indicator of whole-tree growth for bigger individuals. We also confirmed that HW formation is an ontogenetic process managing SW quantity that is continuously and increasingly produced within the crown as the tree grows. This study highlights the effect of growth-mediated ontogenetic changes on the localization of water and carbohydrate storage within a tree, resulting from SW and HW dynamics throughout tree ontogeny.







Similar content being viewed by others
References
Amusant N, Nigg M, Thibaut B, Beauchene J (2014) Diversity of decay resistance strategies of durable tropical woods species: Bocoa prouacencsis Aublet, Vouacapoua americana Aublet, Inga alba (Sw.) Wild. Int Biodeter Biodegr 94:103–108. doi:10.1016/j.ibiod.2014.06.012
Bamber RK (1976) Heartwood, its function and formation. Wood Sci Technol 10:1–8. doi:10.1007/BF00376379
Bamber RK (1987) Sapwood and heartwood. Technical publication (Forestry Commission of New South Wales Wood Technology and Forest Research Division)
Bamber RK, Fukazawa K (1985) Sapwood and heartwood: a review. For Abstr 46:567–580
Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615. doi:10.1046/j.1469-8137.2003.00681.x
Bazot S, Barthes L, Blanot D, Fresneau C (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees-Struct Funct 27:1023–1034. doi:10.1007/s00468-013-0853-5
Brunaux O, Demenois J, Lecoeur N, Guitet S (2009) Directive Regionale d’Aménagement Nord Guyane. Office National des Forêts, Cayenne
Busgen M, Munch E (eds) (1929) The Structure and Life of Forest Trees. Chapman & Hall, UK
Canham CD, LePage PT, Coates KD (2004) A neighborhood analysis of canopy tree competition: effects of shading versus crowding. Can J For Res 34:778–787. doi:10.1139/x03-232
Carbone MS et al (2013) Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytol 200:1145–1155. doi:10.1111/nph.12448
Carrodus BB (1972) Variability in the proportion of heartwood formed in woody stems. New Phytol 71:713–718. doi:10.1111/j.1469-8137.1972.tb01283.x
Cermak J, Kucera J, Bauerle WL, Phillips N, Hinckley TM (2007) Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol 27:181–198
Cirad (2011) Tropix 7, Caractéristiques technologiques de 245 essences tropicales et tempérées, v.7.2.0 edn
Clark JS et al (2011) Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecol Lett 14:1273–1287. doi:10.1111/j.1461-0248.2011.01685.x
Climent J, Chambel MR, Gil L, Pardos JA (2003) Vertical heartwood variation patterns and prediction of heartwood volume in Pinus canariensis Sm. For Ecol Manag 174:203–211. doi:10.1016/S0378-1127(02)00023-3
Drénou C (1988) Etude de l’architecture d’un arbre guyanais: l’angélique, dicorynia guianensis amshoff caesalpiniaceae. Master Thesis, Université de Montpellier II
Fauset S et al (2015) Hyperdominance in Amazonian forest carbon cycling. Nat Commun. doi:10.1038/ncomms7857
Favrichon V (1994) Classification des espèces arborées en groupes fonctionnels en vue de la réalisation d’un modèle de dynamique de peuplement en forêt guyanaise. Rev Ecol-Terre Vie 49:379–403
Fortunel C, Valencia R, Wright SJ, Garwood NC, Kraft NJB (2016) Functional trait differences influence neighbourhood interactions in a hyperdiverse Amazonian forest. Ecol Lett. doi:10.1111/ele.12642
Frey-Wyssling A, Bosshard AA (1959) Cytology of the ray cells in sapwood and heartwood. Holzforschung 13:129–137. doi:10.1515/hfsg.1959.13.5.129
Galván JD, Camarero JJ, Sangüesa-Barreda G, Alla AQ, Gutiérrez E (2012) Sapwood area drives growth in mountain conifer forests. J Ecol 100:1233–1244. doi:10.1111/j.1365-2745.2012.01983.x
Gartner BL (1995) Patterns of Xylem Variation within a Tree and Their Hydraulic and Mechanical Consequences. In: Gartner BL (ed.) Plant Stems. Academic Press, San Diego, p 125–149. doi:http://dx.doi.org/10.1016/B978-012276460-8/50008-4
Godin C, Caraglio Y (1998) A Multiscale Model of Plant Topological Structures. J Theor Biol 191:1–46. doi:10.1006/jtbi.1997.0561
Godin C, Costes E, Caraglio Y (1997) Exploring plant topology structure with the AMAPmod software : an outline. Silva Fenn 31:355–366
Goldstein G, Andrade JL, Meinzer FC, Holbrook NM, Cavelier J, Jackson P, Celis A (1998) Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ 21:397–406. doi:10.1046/j.1365-3040.1998.00273.x
Gourlet-Fleury S, Guehl JM, Laroussinie O (eds.) (2004) Ecology & management of a neotropical rainforest. Lessons drawn from Paracou, a long-term experimental research site in French Guiana. Elsevier, Paris
Hatsch E (1997) Répartition de l’Aubier et acquisition de la forme de la tige chez le chêne sessile (Quercus petraea (Matt) Liebl): Analyse, modélisation et relation avec le développement du houppier
Henriksson J (2001) Differential shading of branches or whole trees: survival, growth, and reproduction. Oecologia 126:482–486. doi:10.1007/s004420000547
Hérault B et al (2011) Functional traits shape ontogenetic growth trajectories of rain forest tree species. J Ecol 99:1431–1440. doi:10.1111/j.1365-2745.2011.01883.x
Hillis WE (1971) Distribution, properties and formation of some wood extractives. Wood Sci Technol 5:272–289. doi:10.1007/BF00365060
Hillis WE (1987) Heartwood and tree exudates. Springer-Verlag,Berlin
Holbrook NM (1995) Stem water storage. In: Gartner BL (ed) Plant stems: physiology and functional morphology. Academic Press, San Diego, pp 151–174
IAWA (1964) Multilingual glossary of terms used in wood anatomy. Verlagsanstalt Buchdruckerei Konkordia, Winterthur
Kampe A, Magel E (2013) New Insights into Heartwood and Heartwood Formation. In: Fromm J (ed.) Cellular Aspects of Wood Formation, vol 20. Plant Cell Monographs. Springer Berlin Heidelberg, p 71–95. doi:10.1007/978-3-642-36491-4_3
Kile G, Tumbull C, Podger F (1981) Effect of `Regrowth Dieback’ on some properties of Eucalyptus obliqua trees. Aust For Res 11:55–62
Koeppen RC (1967) Revision of Dicorynia (Cassieae, Caesalpiniaceae). Brittonia 19:42–61
Långström B, Hellqvist C (1991) Effects of different pruning regimes on growth and sapwood area of Scots pine. For Ecol Manag 44:239–254. doi:10.1016/0378-1127(91)90011-J
Lehnebach R (2015) Variabilité ontogénique du profil ligneux chez les Légumineuses de Guyane Française., Université de Montpellier
Margolis HA, Gagnon RR, Pothier D, Pineau M (1988) The adjustment of growth, sapwood area, heartwood area, and sapwood saturated permeability of balsam fir after different intensities of pruning. Can J For Res 18:723–727. doi:10.1139/x88-110
McDowell N et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. doi:10.1111/j.1469-8137.2008.02436.x
Meinzer FC, James SA, Goldstein G, Woodruff D (2003) Whole-tree water transport scales with sapwood capacitance in tropical forest canopy trees. Plant Cell Environ 26:1147–1155. doi:10.1046/j.1365-3040.2003.01039.x
Meinzer FC, James SA, Goldstein G (2004) Dynamics of transpiration, sap flow and use of stored water in tropical forest canopy trees. Tree Physiol 24:901–909. doi:10.1093/treephys/24.8.901
Meinzer FC, Woodruff DR, Domec JC, Goldstein G, Campanello PI, Gatti MG, Villalobos-Vega R (2008) Coordination of leaf and stem water transport properties in tropical forest trees. Oecologia 156:31–41. doi:10.1007/s00442-008-0974-5
Meinzer FC, Lachenbruch B, Dawson TE (2011) Size- and age-related changes in tree structure and function. Springer, Netherlands
Morataya R, Galloway G, Berninger F, Kanninen M (1999) Foliage biomass–sapwood (area and volume) relationships of Tectona grandis L.F. and Gmelina arborea Roxb.: silvicultural implications. For Ecol Manag 113:231–239. doi:10.1016/S0378-1127(98)00429-0
Nicolini E, Caraglio Y, Pélissier R, Leroy C, Roggy JC (2003) Epicormic BRANCHES: A GROWTH INDICATOR FOR THE TROPICAL FOREST TRee, Dicorynia guianensis Amshoff (Caesalpiniaceae). Ann Bot 92:97–105. doi:10.1093/aob/mcg119
Pérez-Harguindeguy N et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. doi:10.1071/BT12225
Pinto I, Pereira H, Usenius A (2004) Heartwood and sapwood development within maritime pine (Pinus pinaster Ait.) stems. Trees-Struct Funct 18:284–294. doi:10.1007/s00468-003-0305-8
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Rasband WS (2012) ImageJ. US National Institutes of Health, Bethesda
R-Core-Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reyes-García C, Andrade J, Simá JL, Us-Santamaría R, Jackson P (2012) Sapwood to heartwood ratio affects whole-tree water use in dry forest legume and non-legume trees. Trees 26:1317–1330. doi:10.1007/s00468-012-0708-5
Richardson AD et al (2013) Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol 197:850–861. doi:10.1111/nph.12042
Richardson AD et al (2015) Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol 206:590–597. doi:10.1111/nph.13273
Rutishauser E, Barthélémy D, Blanc L, Nicolini E-A (2011) Crown fragmentation assessment in tropical trees: method, insights and perspectives. For Ecol Manag 261:400–407. doi:10.1016/j.foreco.2010.10.025
Sabatier D, Prévost MF (1990) Quelques données sur la composition floristique, et la diversite des peuplements forestiers de guyane francaise. Bois et Forêts des Tropiques 219
Schippers P, Vlam M, Zuidema PA, Sterck F (2015) Sapwood allocation in tropical trees: a test of hypotheses. Funct Plant Biol 42:697–709. doi:10.1071/FP14127
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant Cell Environ 30:236–248. doi:10.1111/j.1365-3040.2006.01623.x
Schulze ED et al (1985) Canopy transpiration and water fluxes in the xylem of the trunk of Larix and Picea trees–a comparison of xylem flow, porometer and cuvette measurements. Oecologia 66:475–483. doi:10.1007/BF00379337
Sillett SC, Van Pelt R, Koch GW, Ambrose AR, Carroll AL, Antoine ME, Mifsud BM (2010) Increasing wood production through old age in tall trees. For Ecol Manag 259:976–994. doi:10.1016/j.foreco.2009.12.003
Spicer R, Gartner BL (2001) The effects of cambial age and position within the stem on specific conductivity in Douglas-fir (Pseudotsuga menziesii) sapwood. Trees 15:222–229. doi:10.1007/s004680100093
Spicer R, Holbrook NM (2005) Within-stem oxygen concentration and sap flow in four temperate tree species: does long-lived xylem parenchyma experience hypoxia? Plant Cell Environ 28:192–201. doi:10.1111/j.1365-3040.2004.01262.x
Stephenson NL, Das AJ, Condit R, Russo SE, Baker PJ, Beckman NG, Coomes DA, Lines ER, Morris WK, Ruger N, Alvarez E, Blundo C, Bunyavejchewin S, Chuyong G, Davies SJ, Duque A, Ewango CN, Flores O, Franklin JF, Grau HR, Hao Z, Harmon ME, Hubbell SP, Kenfack D, Lin Y, Makana JR, Malizia A, Malizia LR, Pabst RJ, Pongpattananurak N, Su SH, Sun IF, Tan S, Thomas D, van Mantgem PJ, Wang X, Wiser SK, Zavala MA (2014) Rate of tree carbon accumulation increases continuously with tree size. Nature 507:90–93
Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability–a review. Wood Fiber Sci 34:587–611
Taylor A, Freitag C, Cadot E, Morrell J (2008) Potential of near infrared spectroscopy to assess hot-water-soluble extractive content and decay resistance of a tropical hardwood. Holz Roh Werkst 66:107–111. doi:10.1007/s00107-007-0214-4
Ter Steege H et al (2006) Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443:444–447 doi:http://www.nature.com/nature/journal/v443/n7110/suppinfo/nature05134_S1.html
Ter Steege H et al (2013) Hyperdominance in the amazonian tree flora. Science. doi:10.1126/science.1243092
Tyree M, Yang S (1990) Water-storage capacity of Thuja, Tsuga and Acer stems measured by dehydration isotherms. Planta 182:420–426. doi:10.1007/BF02411394
van der Sande M, Zuidema P, Sterck F (2015) Explaining biomass growth of tropical canopy trees: the importance of sapwood. Oecologia 177:1145–1155. doi:10.1007/s00442-015-3220-y
Van Pelt R, Sillett SC (2008) Crown development of coastal Pseudotuga menziesii, includinf a conceptual model for tall conifers. Ecol Monogr 78:283–311. doi:10.1890/07-0158.1
Vargas R, Trumbore SE, Allen MF (2009) Evidence of old carbon used to grow new fine roots in a tropical forest. New Phytol 182:710–718. doi:10.1111/j.1469-8137.2009.02789.x
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:259–291. doi:10.1017/S1464793106007007
White DA (1993) Relationships between foliar number and the cross-sectional areas of sapwood and annual rings in red oak (Quercusrubra) crowns. Can J For Res 23:1245–1251. doi:10.1139/x93-159
Whitehead D, Edwards WRN, Jarvis PG (1984) Conducting sapwood area, foliage area, and permeability in mature trees of Piceasitchensis and Pinuscontorta. Can J For Res 14:940–947. doi:10.1139/x84-166
Wirth C, Gleixner G, Heimann M (2009) Old-growth forests: function. Fate and Value, Springer
Wright SJ et al (2010) Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91:3664–3674. doi:10.1890/09-2335.1
Yang KC (1990) The ageing process of sapwood ray parenchyma cells in four woody species. University of British Columbia
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer Series in Wood Science. Springer-Verlag, Berlin. doi:10.1007/978-3-662-22627-8
Acknowledgments
We would like to thank all the members of the professional tree climbers team from the Centre de Formation Professionnelle et de Promotion Agricoles (CFPPA) of Matititi, French Guiana. We also thank logistic and scientific managers of the Paracou field station for their advice and support as well as an anonymous reviewer for his constructive and helpful comments. This work was supported by a grant from the French Agricultural Research and International Cooperation Organization (CIRAD) and funding from the European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Communicated by M. Zwieniecki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lehnebach, R., Morel, H., Bossu, J. et al. Heartwood/sapwood profile and the tradeoff between trunk and crown increment in a natural forest: the case study of a tropical tree (Dicorynia guianensis Amsh., Fabaceae). Trees 31, 199–214 (2017). https://doi.org/10.1007/s00468-016-1473-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1473-7