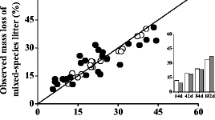
Abstract
Litter decomposition is strongly controlled by litter quality, but the composition of litter mixtures and potential interactions with live plants through root activity may also influence decomposers. In a greenhouse experiment in French Guiana we studied the combined effects of the presence of tropical tree seedlings and of distinct litter composition on mass and nitrogen (N) loss from decomposing litter and on microbial biomass. Different litter mixtures decomposed for 435 days in pots filled with sand and containing an individual seedling from one of four different tree species. We found both additive and negative non-additive effects (NAE) of litter mixing on mass loss, whereas N loss showed negative and positive NAE of litter mixing. If litter from the two tree species, Platonia insignis and Goupia glabra were present, litter mixtures showed more positive and more negative NAE on N loss, respectively. Overall, decomposition, and in particular non-additive effects, were only weakly affected by the presence of tree seedlings. Litter mass loss weakly yet significantly decreased with increasing fine root biomass in presence of Goupia seedlings, but not in the presence of seedlings of any other tree species. Our results showed strong litter composition effects and also clear, mostly negative, non-additive effects on mass loss and N loss. Species identity of tree seedlings can modify litter decomposition, but these live plant effects remain quantitatively inferior to litter composition effects.



Similar content being viewed by others
References
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Arrhenius SP, Langenheim JH (1983) Inhibitory effects of Hymenaea and Copaifera leaf resins on the leaf fungus, Pestalotia subcuticularis. Biochem Syst Ecol 11:361–366
Ayres E, Dromph KM, Bardgett RD (2006) Do plant species encourage soil biota that specialise in the rapid decomposition of their litter? Soil Biol Biochem 38:183–186
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interations with plants and other organisms. Annu Rev Plant Biol 57:233–266
Ball BA, Hunter MD, Kominoski JS, Swan CM, Bradford MA (2008) Consequences of non-random species loss for decomposition dynamics: experimental evidence for additive and non-additive effects. J Ecol 96:303–313
Beck T, Joergensen RG, Kandeler E, Makeschin F, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29:1023–1032
Bonal D, Bosc A, Ponton S, Goret J-Y, Burban B, Gross P, Bonnefond J-M, Elbers JAN, Longdoz B, Epron D, Guehl J-M, Granier A (2008) Impact of severe dry season on net ecosystem exchange in the Neotropical rainforest of French Guiana. Glob Change Biol 14:1917–1933
Cheng W, Kuzyakov Y (2005) Root effects on soil organic matter decomposition. In: Wright S, Zobel R (eds) Roots and soil management: interactions between roots and the soil. American Society of Agronomy, Madison, pp 119–143
Cheng WX, Johnson DW, Fu SL (2003) Rhizosphere effects on decomposition: controls of plant species, phenology, and fertilization. Soil Sci Soc Am J 67:1418–1427
Chigineva NI, Aleksandrova AV, Tiunov AV (2009) The addition of labile carbon alters litter fungal communities and decreases litter decomposition rates. Appl Soil Ecol 42:264–270
Coley PD, Aide TM (1991) Comparison of herbivory and plant defenses in temperate and tropical broad-leaved forests. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New-York, pp 25–49
Coq S, Souquet JM, Meudec E, Cheynier V, Hattenschwiler S (2010) Interspecific variation in leaf litter tannins drives decomposition in a tropical rain forest of French Guiana. Ecology 91:2080–2091
Dijkstra FA, Cheng WX (2007) Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol Lett 10:1046–1053
Dijkstra FA, Cheng WX, Johnson DW (2006) Plant biomass influences rhizosphere priming effects on soil organic matter decomposition in two differently managed soils. Soil Biol Biochem 38:2519–2526
Gallet C, Nilsson MC, Zackrisson O (1999) Phenolic metabolites of ecological significance in Empetrum hermaphroditum leaves and associated humus. Plant Soil 210:1–9
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6:751–765
Gourlet-Fleury S, Guehl JM, Laroussinie O (2004) Ecology and management of a neotropical forest. Lessons drawn from Paracou, a long-term experimental research site in French Guiana. Elsevier, Paris, p 312
Grayston SJ, Prescott CE (2005) Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Biol Biochem 37:1157–1167
Hallam A, Read J (2006) Do tropical species invest more in anti-herbivore defence than temperate species? A test in Eucryphia (Cunoniaceae) in eastern Australia. J Trop Ecol 22:41–51
Hansen RA (1999) Red oak litter promotes a microarthropod functional group that accelerates its decomposition. Plant Soil 209:37–45
Hättenschwiler S, Bracht Jørgensen H (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Hättenschwiler S, Coq S, Barantal S, Handa IT (2010) Leaf traits and decomposition in tropical forests: revisiting some commonly held views and towards a new hypothesis. New Phytol. (in press)
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–2297
Hogberg P, Read DJ (2006) Towards a more plant physiological perspective on soil ecology. Trends Ecol Evol 21:548–554
Hunt HW, Ingham ER, Coleman DC, Elliott ET, Reid CPP (1988) Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology 69:1009–1016
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305
Lokvam J, Braddock JF (1999) Anti-bacterial function in the sexually dimorphic pollinator rewards of Clusia grandiflora (Clusiaceae). Oecologia 119:534–540
Madritch MD, Cardinale BJ (2007) Impacts of tree species diversity on litter decomposition in northern temperate forests of Wisconsin, USA: a multi-site experiment along a latitudinal gradient. Plant Soil 292:147–159
Meier CL, Bowman WD (2008) Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc Natl Acad Sci USA 105:19780–19785
Meier CL, Bowman WD (2010) Chemical composition and diversity influence non-additive effects of litter mixtures on soil carbon and nitrogen cycling: implications for plant species loss. Soil Biol Biochem 42:1447–1454
Negrete-Yankelevich S, Fragoso C, Newton AC, Russell G, Heal OW (2008) Species-specific characteristics of trees can determine the litter macroinvertebrate community and decomposition process below their canopies. Plant Soil 307:83–97
Nilsson MC, Wardle DA, Dahlberg A (1999) Effects of plant litter species composition and diversity on the boreal forest plant-soil system. Oikos 86:16–26
Nilsson MC, Wardle DA, DeLuca TH (2008) Belowground and aboveground consequences of interactions between live plant species mixtures and dead organic substrate mixtures. Oikos 117:439–449
Perez Harguindeguy N, Blundo CM, Gurvich DE, Diaz S, Cuevas E (2008) More than the sum of its parts? Assessing litter heterogeneity effects on the decomposition of litter mixtures through leaf chemistry. Plant Soil 303:151–159
Porazinska DL, Bardgett RD, Blaauw MB, Hunt HW, Parsons AN, Seastedt TR, Wall DH (2003) Relationships at the aboveground-belowground interface: plants, soil biota, and soil processes. Ecol Monogr 73:377–395
Prescott CE, Zabek LM, Staley CL, Kabzems R (2000) Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, forest type, and litter mixtures. Can J For Res Rev Can Rech For 30:1742–1750
Priha O, Smolander A (1996) Microbial biomass and activity in soil and litter under Pinus sylvestris, Picea abies and Betula pendula at originally similar field afforestation sites. Biol Fertil Soils 24:45–51
Scherer-Lorenzen M, Luis Bonilla J, Potvin C (2007) Tree species richness affects litter production and decomposition rates in a tropical biodiversity experiment. Oikos 116:2108–2124
Scheu S (1992) Automated measurement of the respiratory response of soil microcompartments: Active microbial biomass in earthworm faeces. Soil Biol Biochem 24:1113–1118
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford Mark A (2009) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23:627–636
Trinder CJ, Johnson D, Artz RRE (2009) Litter type, but not plant cover, regulates initial litter decomposition and fungal community structure in a recolonising cutover peatland. Soil Biol Biochem 41:651–655
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Acknowledgements
We thank Bruno Buatois, Raphaelle Leclerc and Laurette Sonié for laboratory support, Pascal Petronelli, Jocelyn Cazal, and Audin Patient for their help in seed, seedling and litter collections, Patrick Schevin, Jocelyn Cazal, Nicolas Fanin, Jeremy Cigna, Audin Patient, and Saintano Dufort for help during the final harvest, Benoit Burban for the light measurements in the greenhouse, and Sandra Barantal and Tanya Handa for stimulating discussions. This research was funded through an ATIP research grant from CNRS (SDV) to S.H. Additional support came from the PIR “Amazonie II” from CNRS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Harry Olde Venterink.
Rights and permissions
About this article
Cite this article
Coq, S., Weigel, J., Butenschoen, O. et al. Litter composition rather than plant presence affects decomposition of tropical litter mixtures. Plant Soil 343, 273–286 (2011). https://doi.org/10.1007/s11104-011-0717-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0717-y