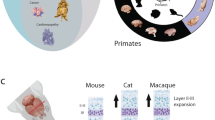
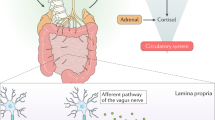
Abstract
Structural and functional elements of biological systems are highly conserved across vertebrates. Many neurological and psychiatric conditions affect both humans and animals. A cross-species approach to the study of brain and behaviour can advance our understanding of human disorders via the identification of unrecognized natural models of spontaneous disorders, thus revealing novel factors that increase vulnerability or resilience, and via the assessment of potential therapies. Moreover, diagnostic and therapeutic advances in human neurology and psychiatry can often be adapted for veterinary patients. However, clinical and research collaborations between physicians and veterinarians remain limited, leaving this wealth of comparative information largely untapped. Here, we review pain, cognitive decline syndromes, epilepsy, anxiety and compulsions, autoimmune and infectious encephalitides and mismatch disorders across a range of animal species, looking for novel insights with translational potential. This comparative perspective can help generate novel hypotheses, expand and improve clinical trials and identify natural animal models of disease resistance and vulnerability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Images courtesy of E. Head, University of Kentucky, USA.

Similar content being viewed by others
References
Lanciotti, R. S. et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286, 2333–2337 (1999).
International Association for the Study of Pain. Terminology. IASP. http://www.iasppain.org/Education/Content.aspx?ItemNumber=1698 (2017).
Wall, P. D. & McMahon, S. B. The relationship of perceived pain to afferent nerve impulses. Trends Neurosci. 9, 254–255 (1986).
Moseley, G. L. & Vlaeyen, J. W. S. Beyond nociception: the imprecision hypothesis of chronic pain. Pain 156, 35–38 (2015).
Loeser, J. D. & Treede, R.-D. The Kyoto protocol of IASP basic pain terminology. Pain 137, 473–477 (2008).
Belin, P., Fillion-Bilodeau, S. & Gosselin, F. The Montreal affective voices: a validated set of nonverbal affect bursts for research on auditory affective processing. Behav. Res. Methods 40, 531–539 (2008).
Briefer, E. F. Vocal expression of emotions in mammals: mechanisms of production and evidence. J. Zool. 288, 1–20 (2012).
Holsti, L. & Grunau, R. E. Initial validation of the behavioral indicators of infant pain (BIIP). Pain 132, 264–272 (2007).
Mosele, M. et al. Psychometric properties of the pain assessment in advanced dementia scale compared to self assessment of pain in elderly patients. Dement. Geriatr. Cogn. Disord. 34, 38–43 (2012).
Reid, J. et al. Development of the short-form Glasgow composite measure pain scale (CMPS-SF) and derivation of an analgesic intervention score. Anim. Welf. 16, 97–104 (2007).
Graubner, C., Gerber, V., Doherr, M. & Spadavecchia, C. Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet. J. 188, 178–183 (2011).
Wright, J. G. Veterinary Anaesthesia (Alexander Eger, Inc., 1941).
Kosek, E. et al. Do we need a third mechanistic descriptor for chronic pain states? Pain 157, 1382–1386 (2016).
Tollison, C. (ed.) in Handbook of Chronic Pain Management (Williams & Wilkins, 1989).
Primason, L., Gleed, R. & Boesch, J. Epidural anaesthesia for treatment of neuropathic pain associated with pelvic limb amputation in a domestic shorthair cat. Vet. Rec. Case Rep. https://doi.org/10.1136/vetreccr-2017-000527 (2017).
Looney, A. Oncology pain in veterinary patients. Top. Companion Anim. Med. 25, 32–44 (2010).
de Grauw, J. C. & van Loon, J. P. Systematic pain assessment in horses. Vet. J. 209, 14–22 (2016).
Klinck, M. P. et al. Translational pain assessment: could natural animal models be the missing link? Pain 158, 1633–1646 (2017).
Hainline, B. et al. International olympic committee consensus statement on pain management in elite athletes. Br. J. Sports Med. 51, 1245–1258 (2017).
Gregory, N. S. et al. An overview of animal models of pain: disease models and outcome measures. J. Pain 14, 1255–1269 (2013).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Facer, P. et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 7, 11 (2007).
O’Neill, J. et al. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol. Rev. 64, 939–971 (2012).
Mogil, J. S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283–294 (2009).
Leal, S. L. & Yassa, M. A. Neurocognitive aging and the hippocampus across species. Trends Neurosci. 38, 800–812 (2015).
Tromp, D., Dufour, A., Lithfous, S., Pebayle, T. & Després, O. Episodic memory in normal aging and Alzheimer disease: insights from imaging and behavioral studies. Ageing Res. Rev. 24, 232–262 (2015).
Espinosa, A. et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J. Alzheimers Dis. 34, 769–780 (2013).
Scheltens, P. et al. Alzheimer’s disease. Lancet 388, 505–517 (2016).
Lane, C. A., Hardy, J. & Schott, J. M. Alzheimer’s disease. Eur. J. Neurol. 25, 59–70 (2018).
Drummond, E. & Wisniewski, T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 133, 155–175 (2017).
Head, E. et al. Beta-amyloid deposition and tau phosphorylation in clinically characterized aged cats. Neurobiol. Aging 26, 749–763 (2005).
Neilson, J. C., Hart, B. L., Cliff, K. D. & Ruehl, W. W. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 218, 1787–1791 (2001).
Hart, B. L. Effect of gonadectomy on subsequent development of age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 219, 51–56 (2001).
Sarasa, M. & Pesini, P. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr. Alzheimer Res. 6, 171–178 (2009).
Rusbridge, C. et al. An aged canid with behavioral deficits exhibits blood and cerebrospinal fluid amyloid beta oligomers. Front. Aging Neurosci. 10, 7 (2018).
Gunn-Moore, D., Kaidanovich-Beilin, O., Gallego Iradi, M. C., Gunn-Moore, F. & Lovestone, S. Alzheimer’s disease in humans and other animals: a consequence of postreproductive life span and longevity rather than aging. Alzheimers Dement. 14, 195–204 (2018).
Youssef, S. A. et al. Pathology of the aging brain in domestic and laboratory animals, and animal models of human neurodegenerative diseases. Vet. Pathol. 53, 327–348 (2016).
Pan, Y. et al. Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br. J. Nutr. 103, 1746–1754 (2010).
Kapl, D. & Rudolphi, K. A. New pharmacologic aspects in the neurologic profile of propentofylline (Karsivan ad us. vet.) [German]. Tierarztl. Prax. Ausg. K. Klientiere Heimtiere 26, 317–321 (1998).
Lemere, C. A. et al. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am. J. Pathol. 165, 283–297 (2004).
Scholtzova, H. et al. Innate immunity stimulation via toll-like receptor 9 ameliorates vascular amyloid pathology in Tg-SwDI mice with associated cognitive benefits. J. Neurosci. 37, 936–959 (2017).
Davis, P. R. et al. Aβ vaccination in combination with behavioral enrichment in aged beagles: effects on cognition, Aβ, and microhemorrhages. Neurobiol. Aging 49, 86–99 (2017).
Gunn-Moore, D., Moffat, K., Christie, L.-A. & Head, E. Cognitive dysfunction and the neurobiology of ageing in cats. J. Small Anim. Pract. 48, 546–553 (2007).
Gunn-Moore, D. A. et al. Ageing changes in cat brains demonstrated by beta-amyloid and AT8-immunoreactive phosphorylated tau deposits. J. Feline Med. Surg. 8, 234–242 (2006).
Grone, B. P. & Baraban, S. C. Animal models in epilepsy research: legacies and new directions. Nat. Neurosci. 18, 339–343 (2015).
Potschka, H., Fischer, A., von Rüden, E.-L., Hülsmeyer, V. & Baumgärtner, W. Canine epilepsy as a translational model? Epilepsia 54, 571–579 (2013).
Podell, M. et al. 2015 ACVIM small animal consensus statement on seizure management in dogs. J. Vet. Intern. Med. 30, 477–490 (2016).
Berendt, M. et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet. Res. 11, 182 (2015).
Bhatti, S. F. M. et al. International veterinary epilepsy task force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet. Res. 11, 176 (2015).
De Risio, L. et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet. Res. 11, 148 (2015).
Hülsmeyer, V.-I. et al. International veterinary epilepsy task force’s current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet. Res. 11, 175 (2015).
Kearsley-Fleet, L., O’Neill, D. G., Volk, H. A., Church, D. B. & Brodbelt, D. C. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet. Rec. 172, 338 (2013).
Licht, B. G. et al. Clinical presentations of naturally occurring canine seizures: similarities to human seizures. Epilepsy Behav. 3, 460–470 (2002).
Patterson, E. E. et al. Canine status epilepticus treated with fosphenytoin: a proof of principle study. Epilepsia 56, 882–887 (2015).
Kwan, P. et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 51, 1069–1077 (2010).
Lohi, H. et al. Expanded repeat in canine epilepsy. Science 307, 81 (2005).
Alves, L. et al. Polymorphisms in the ABCB1 gene in phenobarbital responsive and resistant idiopathic epileptic Border Collies. J. Vet. Intern. Med. 25, 484–489 (2011).
Wielaender, F. et al. Generalized myoclonic epilepsy with photosensitivity in juvenile dogs caused by a defective DIRAS family GTPase 1. Proc. Natl Acad. Sci. USA 114, 2669–2674 (2017).
James, F. M. K. et al. Diagnostic utility of wireless video-electroencephalography in unsedated dogs. J. Vet. Intern. Med. 31, 1469–1476 (2017).
Davis, K. A. et al. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG. Epilepsy Res. 96, 116–122 (2011).
Davis, K. A. et al. Mining continuous intracranial EEG in focal canine epilepsy: relating interictal bursts to seizure onsets. Epilepsia 57, 89–98 (2016).
Sillay, K. A. et al. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 6, 718–726 (2013).
Ung, H. et al. Temporal behavior of seizures and interictal bursts in prolonged intracranial recordings from epileptic canines. Epilepsia 57, 1949–1957 (2016).
Martlé, V. et al. Vagus nerve stimulator placement in dogs: surgical implantation technique, complications, long-term follow-up, and practical considerations. Vet. Surg. 45, 71–78 (2016).
Richter, A., Hamann, M., Wissel, J. & Volk, H. A. Dystonia and paroxysmal dyskinesias: under-recognized movement disorders in domestic animals? A comparison with human dystonia/paroxysmal dyskinesias. Front. Vet. Sci. 2, 65 (2015).
Urkasemsin, G. & Olby, N. J. Canine paroxysmal movement disorders. Vet. Clin. North Am. Small Anim. Pract. 44, 1091–1102 (2014).
de Lahunta, A., Glass, E. N. & Kent, M. Classifying involuntary muscle contractions. Compend. Contin. Educ. Pract. Vet. 28, 516–530 (2006).
Vanhaesebrouck, A. E. et al. A novel movement disorder in related male Labrador Retrievers characterized by extreme generalized muscular stiffness. J. Vet. Intern. Med. 25, 1089–1096 (2011).
Geiger, K. M. & Klopp, L. S. Use of a selective serotonin reuptake inhibitor for treatment of episodes of hypertonia and kyphosis in a young adult Scottish Terrier. J. Am. Vet. Med. Assoc. 235, 168–171 (2009).
Lowrie, M. & Garosi, L. Natural history of canine paroxysmal movement disorders in Labrador retrievers and Jack Russell terriers. Vet. J. 213, 33–37 (2016).
Garosi, L. S., Rossmeisl, J. H., de Lahunta, A., Shelton, G. D. & Lennox, G. Primary orthostatic tremor in Great Danes. J. Vet. Intern. Med. 19, 606–609 (2005).
Forman, O. P. et al. Parallel mapping and simultaneous sequencing reveals deletions in BCAN and FAM83H associated with discrete inherited disorders in a domestic dog breed. PLOS Genet. 8, e1002462 (2012).
Gill, J. L. et al. A canine BCAN microdeletion associated with episodic falling syndrome. Neurobiol. Dis. 45, 130–136 (2012).
Harcourt-Brown, T. Anticonvulsant responsive, episodic movement disorder in a German shorthaired pointer. J. Small Anim. Pract. 49, 405–407 (2008).
Law, T. H. et al. A randomised trial of a medium-chain TAG diet as treatment for dogs with idiopathic epilepsy. Br. J. Nutr. 114, 1438–1447 (2015).
Lowrie, M. et al. The clinical and serological effect of a gluten-free diet in border terriers with epileptoid cramping syndrome. J. Vet. Intern. Med. 29, 1564–1568 (2015).
Lowrie, M. et al. Characterization of paroxysmal gluten-sensitive dyskinesia in Border Terriers using serological markers. J. Vet. Intern. Med. https://doi.org/10.1111/jvim.15038 (2018).
Di Lazzaro, V., Capone, F., Cammarota, G., Di Giuda, D. & Ranieri, F. Dramatic improvement of parkinsonian symptoms after gluten-free diet introduction in a patient with silent celiac disease. J. Neurol. 261, 443–445 (2014).
Merikangas, K. R. et al. Lifetime prevalence of mental disorders in U. S. adolescents: results from the national comorbidity survey replication—adolescent supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49, 980–989 (2010).
Scarlett, J. M., Salman, M. D., New, J. G. & Kass, P. H. Reasons for relinquishment of companion animals in U. S. animal shelters: selected health and personal issues. J. Appl. Anim. Welf. Sci. 2, 41–57 (1999).
Flannigan, G. & Dodman, N. H. Risk factors and behaviors associated with separation anxiety in dogs. J. Am. Vet. Med. Assoc. 219, 460–466 (2001).
Szechtman, H. et al. Obsessive-compulsive disorder: insights from animal models. Neurosci. Biobehav. Rev. 76, 254–279 (2017).
Geller, D. A. et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am. J. Psychiatry 160, 1919–1928 (2003).
Ahmari, S. E. et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 340, 1234–1239 (2013).
March, J. S. et al. The treatment for adolescents with depression study (TADS): long-term effectiveness and safety outcomes. Arch. Gen. Psychiatry 64, 1132–1143 (2007).
Go, Y. Y., Balasuriya, U. B. R. & Lee, C.-K. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin. Exp. Vaccine Res. 3, 58–77 (2014).
Roehrig, J. T. et al. Mutation of the dengue virus type 2 envelope protein heparan sulfate binding sites or the domain III lateral ridge blocks replication in Vero cells prior to membrane fusion. Virology 441, 114–125 (2013).
Tizard, I., Ball, J., Stoica, G. & Payne, S. The pathogenesis of bornaviral diseases in mammals. Anim. Health Res. Rev. 17, 92–109 (2016).
Hoffmann, B. et al. A variegated squirrel bornavirus associated with fatal human encephalitis. N. Engl. J. Med. 373, 154–162 (2015).
Irani, S. R. et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann. Neurol. 69, 892–900 (2011).
Pakozdy, A. et al. Suspected limbic encephalitis and seizure in cats associated with voltage-gated potassium channel (VGKC) complex antibody. J. Vet. Intern. Med. 27, 212–214 (2013).
Prüss, H. et al. Anti-NMDA receptor encephalitis in the polar bear (Ursus maritimus) knut. Sci. Rep. 5, 12805 (2015).
Prüss, H. et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 75, 1735–1739 (2010).
Fang, B. et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 73, 1297–1307 (2016).
Matsuki, N. et al. Prevalence of autoantibody in cerebrospinal fluids from dogs with various CNS diseases. J. Vet. Med. Sci. 66, 295–297 (2004).
Lieberman, D. The Story of the Human Body: Evolution, Health, and Disease (Pantheon Books, New York, 2013).
Ewald, P. W. & Burch, G. E. Evolution of infectious disease (Oxford Univ. Press, 1997).
Salahuddin, P., Rabbani, G. & Khan, R. H. The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cell. Mol. Biol. Lett. 19, 407–437 (2014).
Raichlen, D. A. & Polk, J. D. Linking brains and brawn: exercise and the evolution of human neurobiology. Proc. Biol. Sci. 280, 20122250 (2013).
Marlowe, F. W. Hunter-gatherers and human evolution. Evol. Anthropol. Issues News Rev. 14, 54–67 (2005).
Cotman, C. W. & Berchtold, N. C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301 (2002).
Duzel, E., van Praag, H. & Sendtner, M. Can physical exercise in old age improve memory and hippocampal function? Brain J. Neurol. 139, 662–673 (2016).
Nyberg, J. et al. Cardiovascular fitness and later risk of epilepsy: a Swedish population-based cohort study. Neurology 81, 1051–1057 (2013).
Vivar, C., Potter, M. C. & van Praag, H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 15, 189–210 (2013).
Mieda, M. The roles of orexins in sleep/wake regulation. Neurosci. Res. 118, 56–65 (2017).
Charalambous, M., Shivapour, S. K., Brodbelt, D. C. & Volk, H. A. Antiepileptic drugs’ tolerability and safety – a systematic review and meta-analysis of adverse effects in dogs. BMC Vet. Res. 12, 79 (2016).
Schmidt, F. et al. Detection and quantification of β-Amyloid, Pyroglutamyl Aβ, and Tau in aged canines. J. Neuropathol. Exp. Neurol. 74, 912–923 (2015).
Braak, H., Braak, E. & Strothjohann, M. Abnormally phosphorylated tau protein related to the formation of neurofibrillary tangles and neuropil threads in the cerebral cortex of sheep and goat. Neurosci. Lett. 171, 1–4 (1994).
Nelson, P. T., Greenberg, S. G. & Saper, C. B. Neurofibrillary tangles in the cerebral cortex of sheep. Neurosci. Lett. 170, 187–190 (1994).
Roertgen, K. E. et al. A beta-associated cerebral angiopathy and senile plaques with neurofibrillary tangles and cerebral hemorrhage in an aged wolverine (Gulo gulo). Neurobiol. Aging 17, 243–247 (1996).
Cork, L. C. et al. Neurofibrillary tangles and senile plaques in aged bears. J. Neuropathol. Exp. Neurol. 47, 629–641 (1988).
Bons, N., Rieger, F., Prudhomme, D., Fisher, A. & Krause, K.-H. Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer’s disease? Genes Brain Behav. 5, 120–130 (2006).
Rosen, R. F. et al. Tauopathy with paired helical filaments in an aged chimpanzee. J. Comp. Neurol. 509, 259–270 (2008).
Cramer, P. E. et al. Aging African green monkeys manifest transcriptional, pathological, and cognitive hallmarks of human Alzheimer’s disease. Neurobiol. Aging 64, 92–106 (2018).
Packer, R. A. et al. Characterization and mode of inheritance of a paroxysmal dyskinesia in Chinook dogs. J. Vet. Intern. Med. 24, 1305–1313 (2010).
Ramsey, I. K., Chandler, K. E. & Franklin, R. J. A movement disorder in boxer pups. Vet. Rec. 144, 179–180 (1999).
Kolicheski, A. L. et al. A homozygous PIGN missense mutation in Soft-Coated Wheaten Terriers with a canine paroxysmal dyskinesia. Neurogenetics 18, 39–47 (2017).
Platt, S. R., Stefani, A. D. & Wieczorek, L. Primary orthostatic tremor in a Scottish deerhound. Vet. Rec. 159, 495–496 (2006).
Acknowledgements
Reviewer information
Nature Reviews Neurology thanks Á. Pákozdy, C. Rusbridge and T. Sabin for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing the article. O.D. and B.N.-H. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devinsky, O., Boesch, J.M., Cerda-Gonzalez, S. et al. A cross-species approach to disorders affecting brain and behaviour. Nat Rev Neurol 14, 677–686 (2018). https://doi.org/10.1038/s41582-018-0074-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0074-z
This article is cited by
-
Going beyond established model systems of Alzheimer’s disease: companion animals provide novel insights into the neurobiology of aging
Communications Biology (2023)
-
Autoantibodies in neurological disease
Nature Reviews Immunology (2021)
-
Feline cognitive dysfunction as a model for Alzheimer’s disease in the research of CBD as a potential treatment—a narrative review
Journal of Cannabis Research (2020)