Abstract
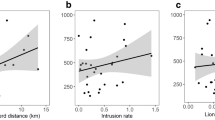
Few studies have experimentally tested the resource dispersion hypothesis (RDH). In this study, I tested whether space use and social organization of Gunnison’s prairie dog responded to changes in the dispersion and abundance of resources. Food manipulations were carried out during the reproductive and nonreproductive seasons across 2 years. Gunnison’s prairie dog adults responded to the experiments by decreasing territory size as food became patchier in space and time. Both males and females modified their home ranges, with no detectable difference between sexes, either prior to or during the experiments. As food became patchier in space and time, the spatial overlap of adults increased, whereas it decreased as food became more evenly dispersed. The average size of a group, defined as those individuals occupying the same territory, did not change significantly as a result of the experiments. Where changes in the composition and size of groups did occur, there was no indication that such changes were sex specific. Results from this study support critical components of the RDH and strongly suggest that patterns of space use and social structure in Gunnison’s prairie dogs are the result of individual responses to resource abundance and distribution.



Similar content being viewed by others
References
Bacon PJ, Ball F, Blackwell P (1991a) Analysis of a model of group territoriality based on the resource dispersion hypothesis. J Theo Biol 148:433–444
Bacon PJ, Ball F, Blackwell P (1991b) A model for territory and group formation in a heterogeneous habitat. J Theo Biol 148:445–468
Baker PJ, Funk SM, Harris S, White PCL (2000) Flexible spatial organization of urban foxes, Vulpes vulpes, before and during an outbreak of sarcoptic mange. Anim Behav 59:127–146
Bakko EB, Nahorniak J (1986) Torpor patterns in captive white-tailed prairie dogs (Cynomys leucurus). J Mamm 67:576–578
Bradbury JW, Vehrencamp SL (1976) Social organization and foraging in emballonurid bats II. A model for the determination of group size. Behav Ecol Socio 2:383–404
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Brown JL (1982) Optimal group-size in territorial animals. J Theo Biol 95:793–810
Carr GM, Macdonald DW (1986) The sociality of solitary foragers-a model based on resource dispersion. Anim Behav 34:1540–1549
Charnov EL, Orians GH, Hyatt K (1976) Ecological implications of resource depression. Am Nat 110:247–259
Crook JH (1965) The adaptive significance of avian social organizations. Symp Zool Soc London 14:181–281
da Silva J, Woodroffe R, Macdonald DW (1993) Habitat, food availability and group territoriality in the European badger, Meles meles. Oecologia 95:558–564
Davies NB, Hartley IR (1996) Food patchiness, territory overlap and social systems: an experiment with dunnocks Prunella modularis. J Anim Ecol 65:837–846
de Waal FB (1997) The chimpanzee’s service economy: food for grooming. Evol Human Behav 18:375–386
Eide NE, Jepsen JU, Prestrud P (2004) Spatial organization of reproductive Arctic foxes Alopex lagopus: responses to changes in spatial and temporal availability of prey. J Anim Ecol 73:1056–1068
Emlen ST, Oring LW (1977) Ecology, sexual selection, and evolution of mating systems. Science 197:215–223
Gill FB, Wolf LL (1975) Foraging strategies and energetics of East-African sunbirds at mistletoe flowers. Am Nat 109:491–510
Haynie ML, Van den Bussche RA, Hoogland JL, Gilbert DA (2003) Parentage, multiple paternity, and breeding success in Gunnison’s and Utah prairie dogs. J Mamm 84:1244–1253
Henzi SP, Barrett L (1999) The value of grooming to female primates. Primates 40:47–59
Hoogland JL (1979) The effect of colony size on individual alertness of prairie dogs (Sciuridae: Cynomys spp.). Anim Behav 27:394–407
Hoogland JL (1981) The evolution of coloniality in white-tailed and black-tailed prairie dogs (Sciuridae: Cynomys leucurus and C. ludovicianus. Ecology 62:252–272
Hoogland JL (1995) The black-tailed prairie dog: social life of a burrowing mammal. University of Chicago Press, Chicago, p 557
Hoogland JL (1999) Philopatry, dispersal, and social organization of Gunnison’s prairie dogs. J Mamm 80:243–251
Janson CH (1992) Evolutionary ecology of primate social structure. In: Smith EA, Winterhalder B (eds) Evolutionary ecology and human behavior. Aldine de Gruyter, New York, pp 95–130
Johnson DDP, Macdonald DW, Newman C, Morecroft MD (2001) Group size versus territory size in group-living badgers: a large-sample field test of the resource dispersion hypothesis. Oikos 95:265–274
Johnson DDP, Kays R, Blackwell PG, Macdonald DW (2002) Does the resource dispersion hypothesis explain group living? TREE 17:63–570
Krebs CJ (1999) Ecological methodology. Addison-Wesley Educational, Reading
Kruuk H, Parish T (1982) Factors affecting population density, group size and territory size of the European badger, Meles meles. J Zool 196:31–39
Kruuk H, Parish T (1987) Changes in the size of groups and ranges of the European badger, Meles meles, in an area in Scotland. J Anim Ecol 56:351–364
Lott DF (1991) Intraspecific variation in the social systems of wild vertebrates. Cambridge University Press, Cambridge
Macdonald DW (1983) The ecology of carnivore social behavior. Nature 301:379–384
Macdonald DW (1984) Carnivore social behavior—does it need patches—reply. Nature 307:390–390
Maher CR, Lott DF (2000) A review of ecological determinants of territoriality within vertebrate species. Am Midl Nat 143:1–29
Myers JP, Connors PG, Pitelka FA (1981) Optimal territory size in the sanderling: compromises in a variable environment. In: Kamil AC, Sargent D (eds) Foraging behavior. Garland STPM, New York, pp 135–158
Nagy KA, Girard IA, Brown TK (1999) Energetics of free-ranging mammals, reptiles, and birds. Ann Rev Nutr 19:247–273
Owens D, Owens M (1996) Social dominance and reproductive patterns in brown hyenas, Hyaena brunnea, of the central Kalahari desert. Anim Behav 51:535–551
Packer C, Scheel D, Pusey AE (1990) Why lions form groups—food is not enough. Am Nat 136:1–19
Rayor LS (1988) Social organization and space-use in Gunnison’s prairie dog. Behav Ecol Socio 22:69–78
Revilla E, Palomares F (2002) Spatial organization, group living and ecological correlates in low-density populations of Eurasian badgers, Meles meles. J Anim Ecol 71:497–512
Sánchez-Prieto CB, Carranza J, Pulido FJ (2004) Reproductive behavior in female Iberian red deer: effects of aggregation and dispersion of food. J Mamm 85:761–767
Seaman DE, Powell RA (1996) An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 77:2075–2085
Seaman DE, Millspaugh JJ, Kernohan BJ, Brundidge GC, Raedke KJ, Gitzen RA (1999) Effects of sample size on kernel home range estimators. J Wildl Manag 63:739–747
Seyfarth RM, Cheney DL (1984) Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308:541–542
Slobodchikoff CN (1984) Resources and the evolution of social behavior. In: Price PW, Slobodchikoff CN, Gaud WS (eds) A new ecology: novel approaches to interactive systems. Wiley, New York, pp 227–251
Stouffer PC, Romagnano LC, Lombardo MP, Hoffenberg AS, Power HW (1988) A case of communal nesting in the European starling. Condor 90:241–245
Tanaka I, Takefushi H (1993) Elimination of external parasites (lice) is the primary function of grooming in free-ranging Japanese macaques. Anthro Sci 101:187–193
Tileston JV, Lechleitner RR (1966) Some comparisons of the black-tailed and white-tailed prairie dogs in north-central Colorado. Am Midl Nat 75:292–316
Travis SE, Slobodchikoff CN (1993) Effects of food resource distribution on the social system of Gunnison’s prairie dog (Cynomys gunnisoni). Can J Zool 71:1186–1192
Travis SE, Slobodchikoff CN, Keim P (1995) Ecological and demographic effects on intraspecific variation in the social system of prairie dogs. Ecology 76:1794–1803
Travis SE, Slobodchikoff CN, Keim P (1996) Social assemblages and mating relationships in prairie dogs: a DNA fingerprint analysis. Behav Ecol 7:95–100
van Schaik CP, Janson CH (2000) Infanticide by males and its implications. Cambridge University Press, Cambridge
Verdolin JL (2007) Resources, not male mating strategies, are a determinant of social structure in Gunnison’s prairie dogs (Cynomys gunnisoni). Behav 144:1361–1382
Verdolin JL, Slobodchikoff CN (2002) Vigilance and predation risk in Gunnison’s prairie dogs (Cynomys gunnisoni). Can J Zool 80:1197–1203
Verdolin JL, Slobodchikoff CN (2009) Resources, not kinship, determine social patterning in the territorial Gunnison’s prairie dog (Cynomys gunnisoni). Ethology 115:59–69
Von Shantz T (1984) Carnivore social behaviour-does it need patches? Nature 307:389–390
Waser PM (1977) Feeding, ranging, and group size in the mangabey Cercocebus albigena. In: Clutton-Brock TH (ed) Primate ecology. Academic, New York, pp 183–222
Waser PM (1988) Resources, philopatry and social interactions among mammals. In: Slobodchikoff CN (ed) Ecology of social behavior. Academic, New York
Woodroffe R, Macdonald DW (2000) Helpers provide no detectable benefits in the European badger (Meles meles). J Zool 250:113–119
Worton B (1995) Using Monte Carlo simulations to evaluate kernel-based home range estimators. J Wildl Manag 59:794–800
Yamagiwa J, Hill D (1998) Intraspecific variation in the social organization of Japanese macaques: past and present scope of field studies in natural habitats. Primates 39:257–273
Acknowledgements
I am grateful to my advisor, Dr. Charles Janson, for his guidance and invaluable contribution to this manuscript. I would also like to thank Drs. Kenneth Armitage, John True, Patricia Wright, and two anonymous reviewers whose comments and suggestions greatly improved this manuscript. For unbelievable field support, veterinary services, and friendship, a heartfelt thank you to Dr. David Washabau. This project could not have been completed without many people dedicating their time and effort, including Bill and Theresa Emig, Carolyn Parker, Perry Crompton, Kristen Hoss, Jessica Hagan, and many others. This study was supported by grants from the American Museum of Natural History, Sigma Xi, and the American Society of Mammalogists. Generous donations of microchips were made by Schlering-Plough and sunflower seeds were kindly provided by Western Organics, Inc. For charitably allowing me use of tomahawk traps, I thank Norris Dodd. Vegetation data were analyzed in the FERTL Lab housed in the Department of Ecology and Evolution, Stony Brook University. Lastly, I thank the City of Flagstaff, Arizona Game and Fish Department and the Arizona State Land Trust Department for all necessary permits. This project was approved by Stony Brook University Animal Care and Use Committee (Permit #1290).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Banks
Rights and permissions
About this article
Cite this article
Verdolin, J.L. Gunnison’s prairie dog (Cynomys gunnisoni): testing the resource dispersion hypothesis. Behav Ecol Sociobiol 63, 789–799 (2009). https://doi.org/10.1007/s00265-009-0712-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0712-y